(E)-octa-1,4-diene | 53793-31-0
中文名称
——
中文别名
——
英文名称
(E)-octa-1,4-diene
英文别名
(E)-1,4-octadiene;1,4-octadiene;octa-1,4t-diene;(E,E)-1,4-Octadien;1,4-Octadien;(4E)-octa-1,4-diene
CAS
53793-31-0
化学式
C8H14
mdl
——
分子量
110.199
InChiKey
HYBLFDUGSBOMPI-BQYQJAHWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:769;771.7;768.7;769
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:8
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:描述:(E)-octa-1,4-diene 、 4-benzyl-2-(4-methoxyphenyl)oxazol-5(4H)-one 在 2,5-二甲基-对苯醌 、 C49H27F5N3O6P 、 bis(dibenzylideneacetone)-palladium(0) 作用下, 以 甲苯 为溶剂, 反应 20.0h, 以85%的产率得到(S)-4-benzyl-2-(4-methoxyphenyl)-4-((R,Z)-octa-5,7-dien-4-yl)oxazol-5(4H)-one参考文献:名称:钯催化的 1,4-二烯不对称烯丙基 C-H 烷基化反应中亲核试剂依赖的 Z/E-和区域选择性摘要:不对称烯丙基烷基化 (AAA) 以使用活性烯丙基底物为特征,在有机合成中具有历史意义。烯丙基 CH 烷基化原则上比经典的烯丙基官能化更具原子和步骤经济性,因此可以被认为是一种变革性的变体。然而,不对称烯丙基 CH 烷基化反应仍然稀少且不发达。在此,我们发现 Pd 催化的 1,4-二烯的不对称烯丙基 CH 烷基化中的 Z/E-和区域选择性高度依赖于亲核试剂的类型。已通过钯-手性亚磷酰胺催化建立了 1,4-二烯与吖内酯的高度立体选择性烯丙基 CH 烷基化反应。该协议在温和的条件下进行,可以适应广泛的底物,提供结构上不同的 α,α-二取代的 α-氨基酸替代物具有高产率和出色的非对映选择性、Z/E-、区域选择性和对映选择性。值得注意的是,该方法为有效合成lepadiformine海洋生物碱提供了关键的手性中间体。对反应机理的实验和计算研究表明,烯丙基 CH 裂解存在一种新的协调质子和双电子转移过程,并揭示DOI:10.1021/jacs.8b13582
-
作为产物:描述:参考文献:名称:Cocker, Wesley; Geraghty, Niall W. A.; Shannon, Patrick V. R., Journal of the Chemical Society. Perkin transactions I, 1984, # 10, p. 2241 - 2244摘要:DOI:
文献信息
-
Palladium-catalyzed synthesis of allylic tertiary amines from vinylic bromides, olefins, and secondary amines作者:Babu A. Patel、Richard F. HeckDOI:10.1021/jo00414a024日期:1978.9
-
BARLUENGA J.; YUS M.; CONCELLON J. M.; BERNARD P., J. CHEM. RES. SYNOP., 1980, NO 2, 41/J. CHEM. RES. MIKROFICHE, 0677-0692作者:BARLUENGA J.、 YUS M.、 CONCELLON J. M.、 BERNARD P.DOI:——日期:——
-
COCKER, W.;GERAGHTY, N. W. A.;MCMURRY, T. B. H.;SHANNON, P. V. R., J. CHEM. SOC. PERKIN TRANS., 1984, N 10, 2245-2254作者:COCKER, W.、GERAGHTY, N. W. A.、MCMURRY, T. B. H.、SHANNON, P. V. R.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
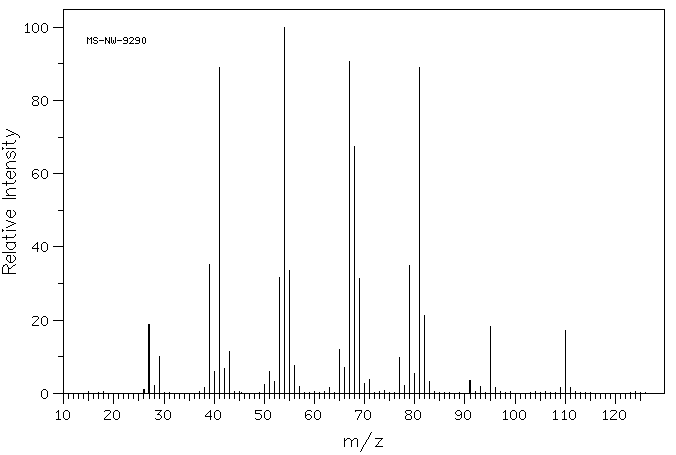
质谱MS
-
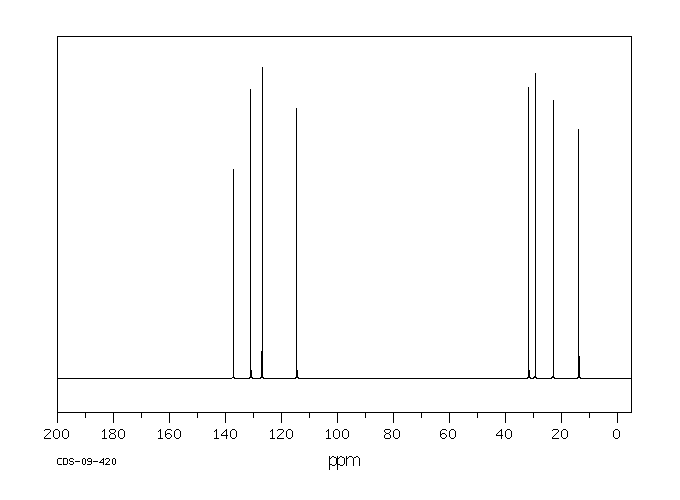
碳谱13CNMR
-
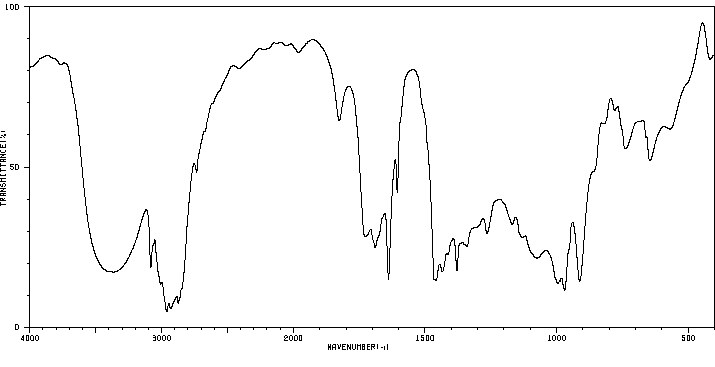
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-