2-benzo[1,3]dioxol-5-yl-4,5-diphenyl-1H-imidazole | 95160-88-6
中文名称
——
中文别名
——
英文名称
2-benzo[1,3]dioxol-5-yl-4,5-diphenyl-1H-imidazole
英文别名
2-(benzo[d][1,3]dioxol-5-yl)-4,5-diphenyl-1H-imidazole;2-<3,4-Methylendioxy-phenyl>-4,5-diphenyl-imidazol;1H-Imidazole, 2-(1,3-benzodioxol-5-yl)-4,5-diphenyl-;2-(1,3-benzodioxol-5-yl)-4,5-diphenyl-1H-imidazole
CAS
95160-88-6
化学式
C22H16N2O2
mdl
——
分子量
340.381
InChiKey
UVLQSZPWZTUDFR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:26
-
可旋转键数:3
-
环数:5.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:47.1
-
氢给体数:1
-
氢受体数:3
上下游信息
反应信息
-
作为反应物:描述:2-benzo[1,3]dioxol-5-yl-4,5-diphenyl-1H-imidazole 在 ammonium acetate 作用下, 以 甲醇 为溶剂, 生成 2-benzo[1,3]dioxol-5-yl-4,6-diphenyl-[1,3,5]triazine参考文献:名称:三芳基咪唑的化学发光反应和三芳基咪唑在氨存在下的光氧化反应中三芳基-s-三嗪的形成摘要:2,4,6-三苯基-s-三嗪 (II) 是由 2,4,5-三苯基咪唑 (I) 在氧气和强碱存在下的化学发光反应或 I 在醇中的光解形成的氨和氧气的存在。II也在含氨的醇中在I的敏化光氧化中形成。发现由I制备的lophyl过氧化氢(V)(已知是I的化学发光反应的中间体之一)与氨反应产生II。化学和光谱证据表明 II 是由 V 产生的,而不是通过二氧杂环丁烷型过氧化物 (VI) 或 N,N'-二苯甲酰苯甲脒 (IV) 产生的。II 和其他几个 2,4,DOI:10.1246/bcsj.44.533
-
作为产物:参考文献:名称:在无催化剂、无溶剂和微波辐射条件下一步合成 2,4,5-三芳基咪唑的简便方法摘要:报道了一种在无催化剂、无溶剂和微波辐射条件下,通过苄基、芳香醛和乙酸铵一步缩合反应合成 2,4,5-三芳基咪唑的绿色高效方法。该方法具有反应时间短(3-5 分钟)、收率高(80-99%)、环境友好、操作方便等显着优点。DOI:10.1080/00397910903043025
文献信息
-
Catalytic procedures for multicomponent synthesis of imidazoles: selectivity control during the competitive formation of tri- and tetrasubstituted imidazoles作者:Dinesh Kumar、Damodara N. Kommi、Narendra Bollineni、Alpesh R. Patel、Asit K. ChakrabortiDOI:10.1039/c2gc35277j日期:——based ionic liquids (organic salts) were investigated for the three component reaction (3-MCR) of 1,2-diketone, aldehyde, and ammonium salts to form 2,4,5-trisubstituted imidazoles and the four component reaction (4-MCR) involving 1,2-diketone, aldehyde, amine and ammonium acetate to form 1,2,4,5-tetrasubstituted imidazoles. The HBF4–SiO2 was found to be the stand out catalyst for both the 3-MCR and不同的氟硼酸衍生的催化剂体系的催化潜力,即。研究了HBF 4水溶液,固体负载的HBF 4,金属四氟硼酸盐(无机盐),固体负载的金属四氟硼酸盐和基于四氟硼酸盐的离子液体(有机盐)对1,2-二酮,醛的三组分反应(3-MCR)和铵盐形成2,4,5-三取代的咪唑,然后进行涉及1,2-二酮,醛,胺和乙酸铵的四组分反应(4-MCR),形成1,2,4,5-四取代的咪唑。发现HBF 4 -SiO 2是3-MCR和4-MCR工艺的杰出催化剂。其次最有效的催化剂是LiBF 4和Zn(BF 4)2分别通过3-MCR和4-MCR形成2,4,5-三取代和1,2,4,5-四取代的咪唑。这是有关4-MCR过程中涉及1,2-二酮,醛,胺和乙酸铵的2,4,5-三取代咪唑竞争形成的未解决问题的第一份报告,并着重指出了催化剂体系在控制催化剂中的影响。选择性形成四取代的咪唑。弱质子酸的金属盐以四氟硼酸盐>高氯酸盐>三氟甲磺酸酯的
-
Direct synthesis of 2,4,5-trisubstituted imidazoles from primary alcohols by diruthenium(<scp>ii</scp>) catalysts under aerobic conditions作者:Saranya Sundar、Ramesh RenganDOI:10.1039/c8ob02785d日期:——report a straightforward synthetic approach to 2,4,5-trisubstituted imidazoles from readily available primary alcohols using arene diruthenium(II) catalysts. Dinuclear arene ruthenium complexes have been synthesized and structurally characterized with the aid of analytical and spectral techniques. A library of 2,4,5-trisubstituted imidazoles was achieved with a yield up to 95% by loading 0.25 mol% of the
-
TMSOTf-catalyzed synthesis of trisubstituted imidazoles using hexamethyldisilazane as a nitrogen source under neat and microwave irradiation conditions作者:Kesatebrhan Haile Asressu、Chieh-Kai Chan、Cheng-Chung WangDOI:10.1039/d1ra05802a日期:——In the process of drug discovery and development, an efficient and expedient synthetic method for imidazole-based small molecules from commercially available and cheap starting materials has great significance. Herein, we developed a TMSOTf-catalyzed synthesis of trisubstituted imidazoles through the reaction of 1,2-diketones and aldehydes using hexamethyldisilazane as a nitrogen source under microwave
-
ZrO<sub>2</sub>-β-cyclodextrin catalyzed synthesis of 2,4,5-trisubstituted imidazoles and 1,2-disubstituted benzimidazoles under solvent free conditions and evaluation of their antibacterial study作者:Yarabhally R. Girish、Kothanahally S. Sharath Kumar、Kuntebommanahalli N. Thimmaiah、Kanchugarakoppal S. Rangappa、Sheena ShashikanthDOI:10.1039/c5ra13891d日期:——
A series of 2,4,5-trisubstituted imidazoles and 1,2-disubstituted benzimidazoles catalyzed by ZrO2-supported-β-cyclodextrin (ZrO2-β-CD) under solvent free conditions have been synthesized and characterized by spectral methods.
-
An eco-friendly, one pot synthesis of tri-substituted imidazoles in aqueous medium catalyzed by RGO supported Au nano-catalyst and computational studies作者:Debopam Sinha、Sudip Biswas、Madhurima Das、Avishek GhatakDOI:10.1016/j.molstruc.2021.130823日期:2021.10protocol for the synthesis of 2,4,5-trisubstituted imidazoles has been achieved with high substrate scope using supported Au nanoparticles. The catalyst can be recovered for the subsequent reactions and reused without any appreciable loss. The utility of this protocol was further explored to synthesis of fused and structurally versatile imidazole also. The synthetic attributes of imidazoles were also demonstrated
表征谱图
-
氢谱1HNMR
-
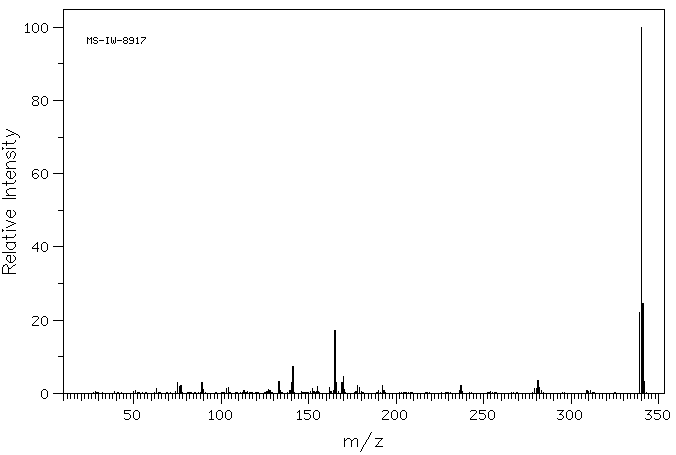
质谱MS
-
碳谱13CNMR
-
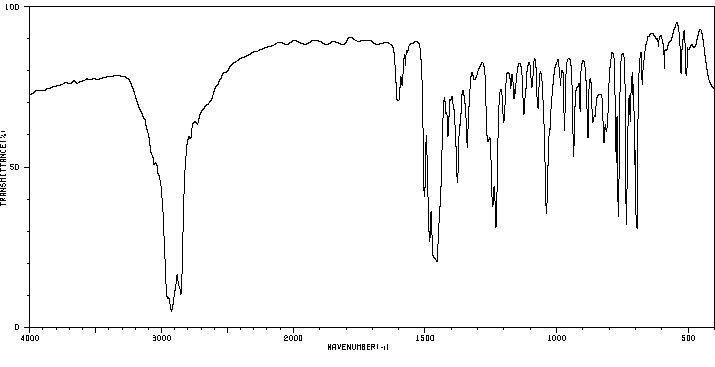
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)