N-methyl-2,4-dinitro-N-phenylaniline | 51226-44-9
中文名称
——
中文别名
——
英文名称
N-methyl-2,4-dinitro-N-phenylaniline
英文别名
(2,4-dinitro-phenyl)-methyl-phenyl-amine;(2,4-Dinitro-phenyl)-methyl-phenyl-amin
CAS
51226-44-9
化学式
C13H11N3O4
mdl
——
分子量
273.248
InChiKey
GRJOQWGGWXHXQQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:20
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:94.9
-
氢给体数:0
-
氢受体数:5
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4-二硝基二苯胺 N-phenyl-2,4-dinitroaniline 961-68-2 C12H9N3O4 259.221 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N1-methyl-4-nitro-N1-phenyl-o-phenylenediamine 861347-82-2 C13H13N3O2 243.265 —— N1-methyl-N1-phenyl-benzene-1,2,4-triyltriamine 48154-07-0 C13H15N3 213.282
反应信息
-
作为反应物:描述:参考文献:名称:Mulder, Recueil des Travaux Chimiques des Pays-Bas, 1906, vol. 25, p. 108摘要:DOI:
-
作为产物:描述:参考文献:名称:1-氯-和1-氟-2,4-二硝基苯与N-甲基苯胺在乙腈中反应的胺盐催化摘要:1-氯-2,4-二硝基苯与N-甲基苯胺反应的二阶速率常数k A随根据方程式k A = k ′+ k ″ [盐]的添加而略有增加。当亲核试剂为苯胺时,氯化物的k ″ / k ′值为8.9,高氯酸盐的值为4.1,远小于先前氯化物的240。N盐酸盐产生的任何加速-甲基苯胺,三甲胺或三乙胺小于高氯酸季铵盐给出的那些。该结果与最近提出的用于乙腈中苯胺氯化反应的卤离子催化的机理相吻合。DOI:10.1039/p29840000681
文献信息
-
Aryl-Diadamantyl Phosphine Ligands in Palladium-Catalyzed Cross-Coupling Reactions: Synthesis, Structural Analysis, and Application作者:Ádám Sinai、Dániel Cs. Simkó、Fruzsina Szabó、Attila Paczal、Tamás Gáti、Attila Bényei、Zoltán Novák、András KotschyDOI:10.1002/ejoc.201901834日期:2020.3.8Bulky diadamantyl aryl phoshine ligands were synthesized and utilized in Buchwald‐Hartwig coupling reactions of sterically demanding ortho‐substituted aryl chlorides. The ligands also showed enhanced catalytic activity in the coupling of tosyl hydrazones and aryl halides.
-
Steric Effects in Nucleophilic Aromatic Substitution reactions with aromatic amines 12th communication on nucleophilic aromatic substitution reactions作者:Walter Eggimann、Peter Schmid、Heinrich ZollingerDOI:10.1002/hlca.19750580132日期:——of over 20 000. The reaction of aniline with 4-X-2,6-dinitrochlorobenzenes is subject to considerably larger para-substituent effects than the corresponding reactions with N-methylaniline. These results are interpreted in terms of two effects: (i) A primary steric effect, which renders the approach of N-methylaniline to the substrate difficult. (ii) A shift towards earlier, more reactant-like transition苯胺和N-甲基苯胺与各种取代的硝基氯苯在乙腈中的S N Ar反应动力学表明,中间体σ-络合物的形成决定了速率。苯胺和N-甲基苯胺反应的速率常数之比(k A / k M)随着6位取代基尺寸的增加而增加。用苦基氯ķ甲/ ķ中号达到的超过一个值20000 。苯胺与4-X-2,6- dinitrochlorobenzenes反应受到相当大的第-取代基的作用要比与N-甲基苯胺的相应反应要大。这些结果可以用两种效应来解释:(i)主要的空间效应,这使N-甲基苯胺难以接近底物。(ii)由主要的空间效应引起的向更早的,更像反应物的过渡态结构的转变。在早期过渡态中,预计衬底中吸电子替代物的活化能力相对较小。缓慢的N-甲基苯胺反应的早期过渡态和快速苯胺反应的晚期过渡态明显与基于Hammond假设的预期相矛盾。
-
Reaction Products of Activated Aromatic and Heteroaromatic Chlorides with <i>N,N</i>-Disubstituted Formamides作者:Zdzisław Brzozowski、Jarosław SławińskiDOI:10.1080/00397910903136118日期:2010.5.11N-disubstituted aromatic amines (3a–g) was obtained in very good yield by the reaction of adequate doubly activated aromatic or heteroaromatic halides (1a–e) with N,N-disubstituted formamides (2a–c). Analogously, starting from 4-chloro-3-pyridinesulfonamide, the appropriate 4-dialkylamino-1H +-pyridinium-3-(N-formyl)sulfonamidates (5a,b) were obtained in 52–60% yield. Mechanisms of the reactions are discussed
-
Effects of the Nucleophile Structure on the Mechanisms of Reaction of 1-Chloro-2,4-dinitrobenzene with Aromatic Amines in Aprotic Solvents作者:Norma Sbarbati Nudelman、Cecilia E. Silvana Alvaro、Monica Savini、Viviana Nicotra、Jeannette YankelevichDOI:10.1135/cccc19991583日期:——
The kinetics of reactions of 1-chloro-2,4-dinitrobenzene with aniline and several substituted aromatic amines, B, in toluene shows a quadratic dependence of the second-order rate constant,
k A, on [B], which is preserved even in the presence of increasing amounts of dimethylaniline, while the reaction withN -methylaniline shows a linear dependence ofk Avs [B]. All these results are interpreted by the "dimer nucleophile" mechanism, and confirmed by the effects of a non-nucleophilic hydrogen bond acceptor tertiary amine which show the relevance of the structure of the nucleophile and the role of mixed aggregates in defining the mechanisms of aromatic nucleophilic substitutions with amines in aprotic solvents.1-氯-2,4-二硝基苯与苯胺和几种取代芳香胺B在甲苯中反应的动力学表明,二级速率常数kA对[B]呈二次依赖关系,即使在存在增加的二甲基苯胺的情况下,这种关系仍然存在,而与N-甲基苯胺的反应则表现出kA与[B]的线性关系。所有这些结果都被解释为“二聚核苷酸互变机制”,并通过非核苷酸氢键受体三级胺的影响得到确认,这表明了核苷酸的结构以及混合聚集体在无极性溶剂中定义芳香族亲核取代反应机制的作用。 -
Reitzenstein, Journal fur praktische Chemie (Leipzig 1954), 1903, vol. <2>68, p. 257作者:ReitzensteinDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
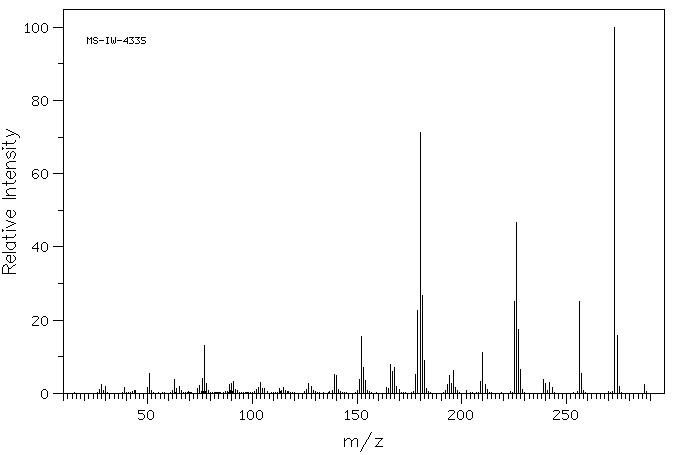
质谱MS
-
碳谱13CNMR
-
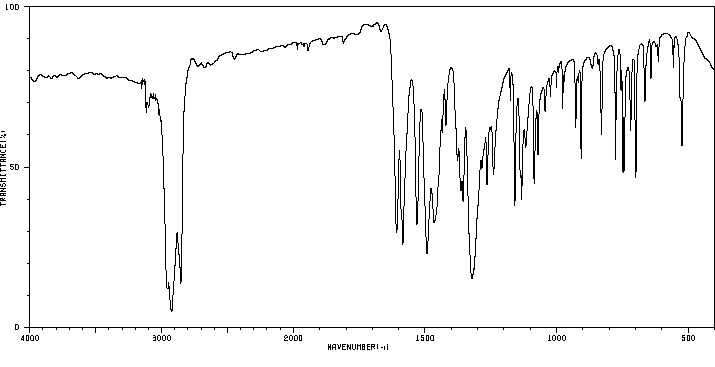
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫