5-氨基-4-氰基(1-2,4,6-三氯苯甲基)吡唑 | 79002-96-3
中文名称
5-氨基-4-氰基(1-2,4,6-三氯苯甲基)吡唑
中文别名
5-氨基-1-(2,4,6-三氯苯基)-1H-吡唑-4-甲腈
英文名称
5-amino-1-(2,4,6-trichlorophenyl)-1H-pyrazole-4-carbonitrile
英文别名
5-amino-4-cyano-1-(2,4,6-trichlorophenyl)pyrazole;5-amino-1-(2,4,6-trichlorophenyl)pyrazole-4-carbonitrile
CAS
79002-96-3
化学式
C10H5Cl3N4
mdl
——
分子量
287.535
InChiKey
XLVSDPGXTLGTAE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:187-195°C
-
沸点:187 °C
-
稳定性/保质期:
远离氧化物、光、热和酸。
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:17
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:67.6
-
氢给体数:1
-
氢受体数:3
安全信息
-
储存条件:存放在密封容器内,并放置在阴凉、干燥处。请将储存地点远离氧化剂,并在避光条件下于室温下保存。
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (+/-)-4-cyano-5-(4-methyl-2-oxo-azetidin-1-yl)-1-(2,4,6-trichlorophenyl)pyrazole 90630-01-6 C14H9Cl3N4O 355.611
反应信息
-
作为反应物:描述:5-氨基-4-氰基(1-2,4,6-三氯苯甲基)吡唑 在 硫酸 、 sodium ethanolate 作用下, 以 乙醇 为溶剂, 反应 18.5h, 生成 6-methyl-1-(2,4,6-trichloro-phenyl)-1,5-dihydro-pyrazolo[3,4-d]pyrimidin-4-one参考文献:名称:Synthesis and Biological Evaluation of 1-Aryl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidin-4-one Inhibitors of Cyclin-Dependent Kinases摘要:Using a high-throughput screening strategy, a series of 1-aryl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidin-4-ones was identified that inhibit the cyclin-dependent kinase (CDK) 4/cyclin D1 complex-mediated phosphorylation of a protein substrate with IC(50)s in the low micromolar range. On the basis of preliminary structure-activity relationships (SAR), a model was proposed in which these inhibitors occupy the ATP-binding site of the enzyme, forming critical hydrogen bonds to the same residue (Val96) to which the amino group in ATP is presumed to bind. X-ray diffraction studies on a later derivative bound to CDK2 support this binding mode. Iterative cycles of synthesis and screening lead to a novel series of potent, CDK2-selective 6-(arylmethyl)pyrazolopyrimidinones. Placement of a hydrogen-bond donor in the meta-position on the 6-arylmethyl group resulted in approximately 100-fold increases in CDK4 affinity, giving ligands that were equipotent inhibitors of CDK4 and CDK2. These compounds exhibit antiproliferative effects in the NCI HCT116 and other cell lines. The potency of these antiproliferative effects is enhanced in anilide derivatives and translates into tumor growth inhibition in a mouse xenograft model.DOI:10.1021/jm020455u
-
作为产物:参考文献:名称:Pyrazolopyrimidines as CRF antagonists摘要:以下为该化合物的中文翻译及其药用酸盐: 该化合物的化学式为:其中A为NR1R2、CR1R2R11、或C(═CR1R12)R2、NHCR1R2R11、OCR1R2R11、SCR1R2R11、NHNR1R2、CR2R11NHR1、CR2R11OR1、CR2R11SR1或C(O)R2;其中其余变量定义如下,用于治疗炎症性疾病。公开号:US06218397B1
文献信息
-
A Facile One-pot Synthesis of 1-Arylpyrazolo[3,4-d]Pyrimidin- 4-ones作者:Xiaohong Zhang、Qiulian Lin、Ping ZhongDOI:10.3390/molecules15053079日期:——One pot synthesis of 1-arylpyrazolo[3,4-d]pyrimidin-4-ones by the reaction of 5-amino-N-substituted-1H-pyrazole-4-carbonitrile with different lower aliphatic acids in the presence of POCl3has been developed. The structures of all the title compounds have been confirmed by IR, 1H-NMR, 13C-NMR, and elemental analyses. Moreover, the structures of one of these compounds, 2c, was confirmed by single-crystal X-ray diffraction.
-
5-Acylamino-4-cyano-1-phenylpyrazole derivatives and use as herbicides申请人:May & Baker Limited公开号:US04459150A1公开(公告)日:1984-07-10New acylamino-N-phenylpyrazole derivatives of the formula: ##STR1## [wherein R.sup.1 represents R.sup.8 C(.dbd.O)--(wherein R.sup.8 represents H, C.sub.1-7 alkyl or C.sub.1-4 alkoxy optionally substituted by C.sub.1-4 alkoxy, C.sub.2-5 alkoxycarbonyl or halogen, C.sub.3-4 alkenyloxy, C.sub.3-6 cycloalkyl optionally substituted by CH.sub.3 or C.sub.2 H.sub.5, or phenoxy, R.sup.2 represents H or R.sup.8 C(.dbd.O)--, or R.sup.1 and R.sup.2 together represent --CO--(CR.sup.a R.sup.b).sub.m --CO--, R.sup.3 represents F, Cl, Br, C.sub.1-4 alkyl optionally substituted by halogen, or C.sub.2-4 alkenyl, R.sup.4 represents F, Cl, Br, NO.sub.2, CH.sub.3 or C.sub.2 H.sub.5 and R.sup.5, R.sup.6 and R.sup.7 represent H, F, Cl, Br, NO.sub.2, CH.sub.3 or C.sub.2 H.sub.5, or R.sup.4 and R.sup.5 each represent Cl and R.sup.3, R.sup.6 and R.sup.7 each represent H, R.sup.a and R.sup.b represent H or C.sub.1-4 alkyl, and m is 2 or 3] possess useful herbicidal properties.新的酰胺基-N-苯基吡唑衍生物的化学式为:##STR1## [其中 R.sup.1 代表 R.sup.8 C(.dbd.O)--(其中 R.sup.8 代表 H、C.sub.1-7 烷基或C.sub.1-4 烷氧基,可选择地被C.sub.1-4 烷氧基、C.sub.2-5 烷氧羰基或卤素取代,C.sub.3-4 烯氧基,C.sub.3-6 环烷烷基,可选择地被CH.sub.3 或C.sub.2 H.sub.5 取代,或苯氧基,R.sup.2 代表 H 或 R.sup.8 C(.dbd.O)--,或R.sup.1 和R.sup.2 共同代表--CO--(CR.sup.a R.sup.b).sub.m --CO--,R.sup.3 代表 F、Cl、Br、可选择地被卤素取代的C.sub.1-4 烷基,或C.sub.2-4 烯基,R.sup.4 代表 F、Cl、Br、NO.sub.2、CH.sub.3 或C.sub.2 H.sub.5,而 R.sup.5、R.sup.6 和 R.sup.7 代表 H、F、Cl、Br、NO.sub.2、CH.sub.3 或C.sub.2 H.sub.5,或者 R.sup.4 和 R.sup.5 各自代表 Cl,而 R.sup.3、R.sup.6 和 R.sup.7 各自代表 H,R.sup.a 和 R.sup.b 代表 H 或 C.sub.1-4 烷基,m 为 2 或 3] 具有有用的除草特性。
-
Herbicidal 5-halo-1-halophenyl-1H-pyrazole-4-carbonitriles申请人:Eli Lilly and Company公开号:US04563210A1公开(公告)日:1986-01-07The present invention is directed to herbicidal compounds of the formula ##STR1## wherein each R.sup.1 independently is halogen; R.sub.2 is halo or trifluoromethyl; and n is 1-5; with the provisos that when n is 1, R.sup.1 is other than fluorine, and when n is 2 and each R.sup.1 is chlorine, at least one R.sup.1 is located at a para or ortho position on the phenyl ring.
-
Herbicidal 5-amino-4-cyano-1-phenylpyrazoles申请人:May & Baker Limited公开号:US04629495A1公开(公告)日:1986-12-16Herbicidal compounds of the formula: ##STR1## wherein A represents R.sup.1 R.sup.2 N-- (wherein R.sup.1 represents C.sub.1-8 alkyl or C.sub.2-8 alkenyl or alkynyl unsubstituted or substituted by CN, OH, C.sub.1-6 alkoxy, carboxy, C.sub.2-9 alkoxycarbonyl, aminocarbonyl optionally substituted by C.sub.1-8 alkyl or C.sub.2-8 alkenyl, C.sub.1-8 alkoxyaminocarbonyl, C.sub.1-8 alkanesulphonamidocarbonyl, --C(.dbd.O)Het, where Het represents a nitrogen-containing heterocyclic group, or one or more halogen atoms or R.sup.1 represents C.sub.3-6 cycloalkyl optionally substituted by C.sub.1-4 alkyl and R.sup.2 represents H or R.sup.1, or R.sup.1 represents C.sub.1-4 alkylthio and R.sup.2 represents H, or A represents R.sup.p (R.sup.q)--C.dbd.N-- (wherein R.sup.p represents C.sub.1-4 alkoxy or amino substituted by one or two C.sub.1-4 alkyl groups and R.sup.q represents H or C.sub.1-4 alkyl) or A represents 2-oxo-azetidin-1-yl, 2-oxo-pyrrolidin-1-yl or 2-oxo-piperidin-1-yl optionally substituted by C.sub.1-6 alkyl or A represents open-chain alkenylcarbonylamino and B represents phenyl substituted in the 2- position by F, Cl, Br, NO.sub.2, Me or Et and in the 4- position by F, Cl, Br, C.sub.1-4 (optionally substituted by halogen) or C.sub.2-4 alkenyl and alkynyl and optionally substituted in the 3-, 5- and 6- positions by F, Cl, Br, NO.sub.2, Me or Et, or B represents 2,3-dichlorophenyl, and salts thereof.式子为:##STR1## 其中A代表R.sup.1 R.sup.2 N--(其中R.sup.1代表C.sub.1-8烷基或C.sub.2-8烯基或炔基,未取代或取代CN、OH、C.sub.1-6烷氧基、羧基、C.sub.2-9烷氧基羰基、氨基羰基,可选地取代C.sub.1-8烷基或C.sub.2-8烯基、C.sub.1-8烷氧基氨基羰基、C.sub.1-8烷基磺酰胺基羰基,--C(.dbd.O)Het,其中Het代表含氮杂环基团,或一个或多个卤素原子,或R.sup.1代表可选地取代C.sub.1-4烷基的C.sub.3-6环烷基,R.sup.2代表H或R.sup.1,或R.sup.1代表C.sub.1-4烷基硫基,R.sup.2代表H,或A代表R.sup.p(R.sup.q)--C.dbd.N--(其中R.sup.p代表取代一个或两个C.sub.1-4烷基的C.sub.1-4烷氧基或氨基,R.sup.q代表H或C.sub.1-4烷基),或A代表2-氧代-氮杂环戊酰胺基、2-氧代-吡咯烷酰胺基或2-氧代-哌啶酰胺基,可选地取代C.sub.1-6烷基或A代表开链烯基羰基氨基,B代表在2-位取代F、Cl、Br、NO.sub.2、Me或Et的苯基,且在4-位取代F、Cl、Br、C.sub.1-4(可选地取代卤素)或C.sub.2-4烯基和炔基,可选地在3-、5-和6-位取代F、Cl、Br、NO.sub.2、Me或Et,或B代表2,3-二氯苯基,以及其盐。
-
N-phenylpyrazole derivatives申请人:MAY & BAKER LIMITED公开号:EP0034945A2公开(公告)日:1981-09-02N-Phenylpyrazole derivatives of the formula:- (wherein each of R5 and R6 represents a C1-C4 alkyl or alkoxy radical, a trifluoromethyl, trifluoromethoxy, nitro, cyano or primary amino radical, or a fluorine, chlorine or bromine atom, each of R', R' and R9 represents a hydrogen atom, a C1-C4 alkyl or alkoxy radical, a trifluoromethyl, trifluoromethoxy, nitro, cyano or primary amino radical or a fluorine, chlorine or bromine atom, or R5, R7, R' and R9 each represents a hydrogen atom and R6 represents a trifluoromethoxy or trifluoromethyl radical, and R10 represents a cyano radical or substituted carbamoyl radical -CONHR", wherein R" represents a methyl or ethyl radical) have been found to possess useful herbicidal properties. All such N-phenylpyrazole derivatives with the exception of 5-amino-4-cyano-1-(2,4-dichlorophenyl)pyrazole and 5-amino-4-cyano-1- (4-chloro-2-methylphenyl)pyrazole are new compounds. Herbicidal compositions containing such N-phenylpyrazole derivatives are described and also processes for the prepararation of the new compounds.式中的 N-苯基吡唑衍生物 (其中R5和R6各自代表C1-C4烷基或烷氧基、三氟甲基、三氟甲氧基、硝基、氰基或伯氨基或氟、氯或溴原子,R'、R'和R9各自代表氢原子、C1-C4烷基或烷氧基、三氟甲基、三氟甲氧基、硝基、氰基或伯氨基或氟、R5、R7、R'和 R9 各代表一个氢原子,R6 代表三氟甲氧基或三氟甲基基,R10 代表氰基或取代的氨基甲酰基 -CONHR",其中 R "代表甲基或乙基)已被发现具有有用的除草特性。除 5-氨基-4-氰基-1-(2,4-二氯苯基)吡唑和 5-氨基-4-氰基-1-(4-氯-2-甲基苯基)吡唑外,所有这些 N-苯基吡唑衍生物都是新化合物。本文介绍了含有此类 N-苯基吡唑衍生物的除草组合物,以及制备这些新化合物的工艺。
表征谱图
-
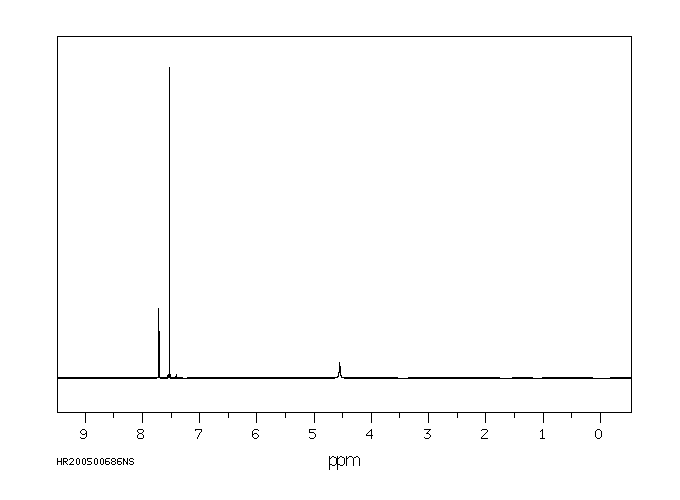
氢谱1HNMR
-
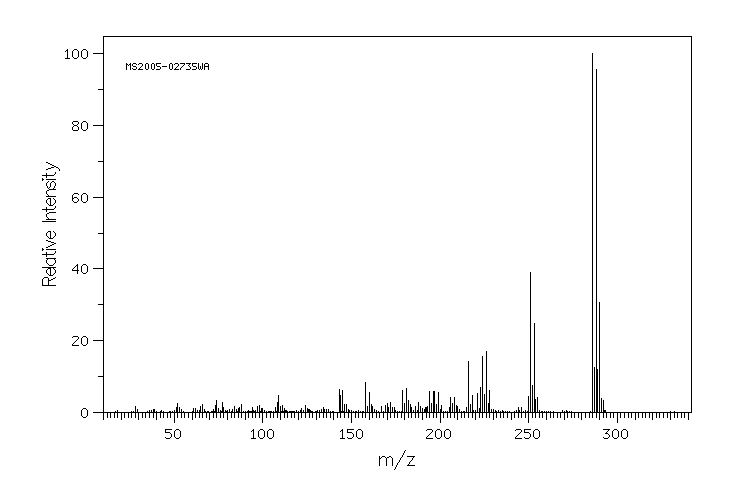
质谱MS
-
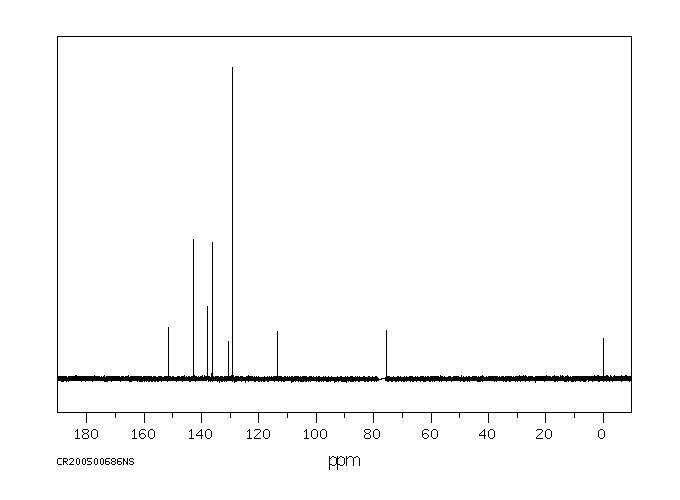
碳谱13CNMR
-
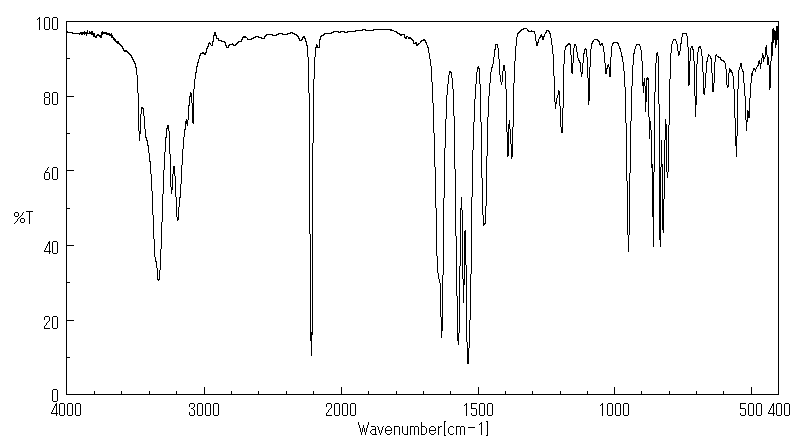
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)