6-乙基四氢-2H-吡喃-2-酮 | 3301-90-4
中文名称
6-乙基四氢-2H-吡喃-2-酮
中文别名
——
英文名称
6-ethyl-tetrahydro-2H-pyran-2-one
英文别名
5-ethyl-δ-valerolactone;5-heptanolide;6-ethyl-tetrahydro-pyran-2-one;6-Aethyl-tetrahydro-pyran-2-on;5-ethyl-5-hydroxyvaleric acid lactone;6-ethyltetrahydro-2H-pyran-2-one;6-ethyloxan-2-one
CAS
3301-90-4
化学式
C7H12O2
mdl
——
分子量
128.171
InChiKey
JFVQYQDTHWLYHG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:68-69 °C(Press: 1 Torr)
-
密度:0.974±0.06 g/cm3(Predicted)
-
LogP:0.940 (est)
-
保留指数:1147
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2932999099
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2H-吡喃-2-酮,6-乙基四氢-,(S)- (S)-(-)-5-ethyl-δ-valerolactone 108943-44-8 C7H12O2 128.171 —— ethyl 5-ethoxyheptanoate —— C11H22O3 202.294
反应信息
-
作为反应物:描述:6-乙基四氢-2H-吡喃-2-酮 在 palladium on activated carbon 、 W(OTf)6 、 氢气 作用下, 以 neat (no solvent) 为溶剂, 135.0 ℃ 、101.33 kPa 条件下, 反应 12.0h, 以92%的产率得到庚酸参考文献:名称:金属三氟甲磺酸酯促进内酯氢解成羧酸的综合研究:从合成和机理的角度摘要:内酯直接氢解为羧酸(即不接触羰基的C烷氧基-O键的氢解)通常是困难的,因为当前使用布朗斯台德酸作为催化剂的策略通常需要苛刻的条件,例如高温和高温H 2压力。在此,我们报告了已开发的无溶剂催化转化方法,其中W(OTf)6被认为可以促进氢解过程。该策略可以在特别温和的条件下(例如,反应温度<150°C和1 atm H 2的条件下)有效地将内酯氢化为羧酸),并显示出较宽的基材范围。另外,该催化方案可以进一步应用于作为可再生聚合物的聚羟基链烷酸酯的氢解成相应的直链羧酸。随后进行了广泛的机理研究,密度泛函理论计算揭示了一种反应模式,包括在W(OTf)6的帮助下C═O键的完全裂解催化剂。此外,通过电喷雾电离质谱已成功检测到该机理中产生的关键中间体,即具有OTf部分的moiety。通过与布朗斯台德酸催化体系的比较,研究证实了OTf部分的存在可以显着降低与重排和消除过程相关的障碍。同时,重点放在阴离子发挥DOI:10.1021/acscatal.7b01569
-
作为产物:描述:参考文献:名称:Addition of Hydrogen Bromide to trans-4-Heptenoic Acid摘要:DOI:10.1021/ja01638a005
文献信息
-
Stereoselective Synthesis of δ-Lactones from 5-Oxoalkanals via One-Pot Sequential Acetalization, Tishchenko Reaction, and Lactonization by Cooperative Catalysis of Samarium Ion and Mercaptan作者:Jue-Liang Hsu、Jim-Min FangDOI:10.1021/jo016058t日期:2001.12.1sequence of acetalization, Tishchenko reaction and lactonization. The deliberative use of mercaptan, by comparison with alcohol, is advantageous to facilitate the catalytic cycle. The reaction mechanism and stereochemistry are proposed and supported by some experimental evidence. Such samarium ion/mercaptan cocatalyzed reactions show the feature of remote control, which is applicable to the asymmetric synthesis
-
[EN] KETAL ESTERS OF ANHYDROPENTITOLS AND USES THEREOF<br/>[FR] CÉTO-ESTERS D'ANHYDROPENTITOLS ET LEURS UTILISATIONS申请人:XLTERRA INC公开号:WO2010138842A1公开(公告)日:2010-12-02The present disclosure relates to the preparation of ketal compounds from anhydropentitols and oxocarboxylates; derivatives, homopolymers, and copolymers thereof; and various compositions, formulations, and articles derived therefrom.
-
Calcium(II)- and Triflimide-Catalyzed Intramolecular Hydroacyloxylation of Unactivated Alkenes in Hexafluoroisopropanol作者:Chenxiao Qi、Shengwen Yang、Vincent Gandon、David LebœufDOI:10.1021/acs.orglett.9b02705日期:2019.9.20hydroacyloxylation of unactivated alkenes, offering a streamlined access to relevant γ-lactones, which features the utilization of either a calcium(II) salt or triflimide as a catalyst in hexafluoroisopropanol. This method could be applied to the synthesis of natural products and the late-stage functionalization of natural and bioactive molecules. Additionally, DFT computations were used to elucidate the twist
-
One-Pot Bi(OTf)3-Catalyzed Oxidative Deprotection of tert-Butyldimethyl Silyl Ethers with TEMPO and Co-Oxidants作者:Jean-Michel Vatèle、Bogdan BarnychDOI:10.1055/s-0030-1260980日期:2011.9A sequential one-pot synthesis for the oxidation of primary and secondary tert-butyldimethylsilyl (TBDMS) ethers, using catalytic amounts of metal triflates and TEMPO in combination with PhIO or PhI(OAc)2 in THF or acetonitrile, is described. Acid-sensitive protecting groups such as methylidene, isopropylidene, acetals, and Boc are unaffected under the reaction conditions. Another feature of this procedure
-
THIOPYRANOSE COMPOUND AND METHOD FOR PRODUCING SAME申请人:FUJIFILM Corporation公开号:US20160355497A1公开(公告)日:2016-12-08There is provided a production method of a thiopyranose compound represented by the following Formula (2) by reacting a compound represented by the following Formula (1) with a sulfur compound. X represents a leaving group. A represents an oxygen atom or a sulfur atom. Further, each of R 1A to R 4B , R 1B to R 4B , and R 5 represents a hydrogen atom or a specific substituent.
表征谱图
-
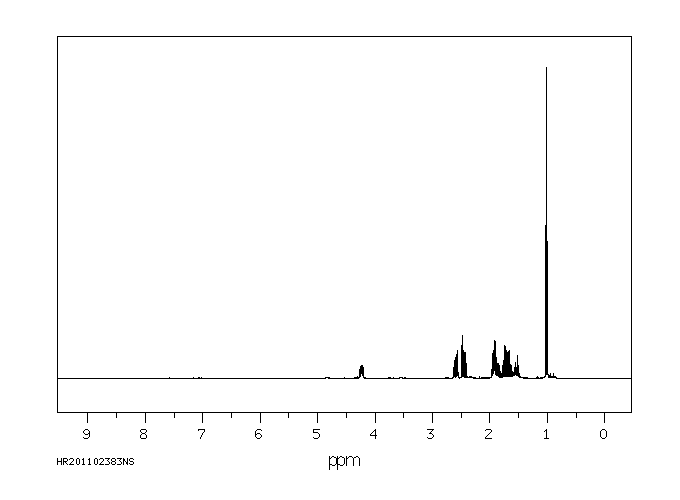
氢谱1HNMR
-
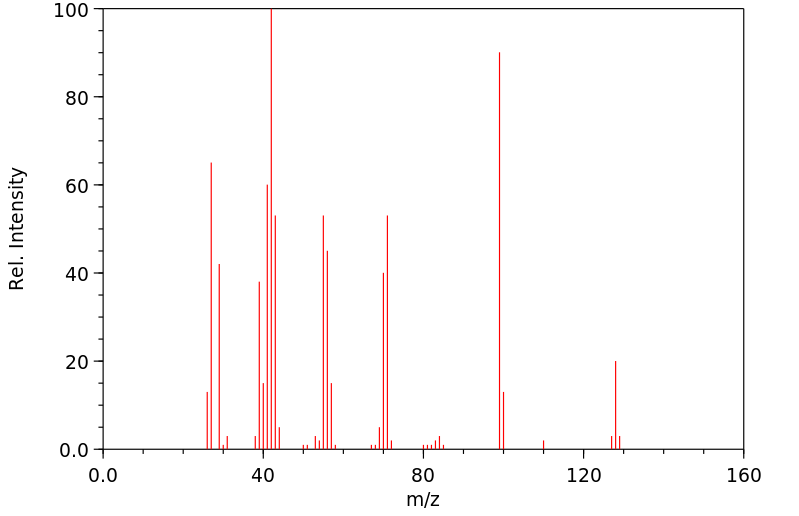
质谱MS
-
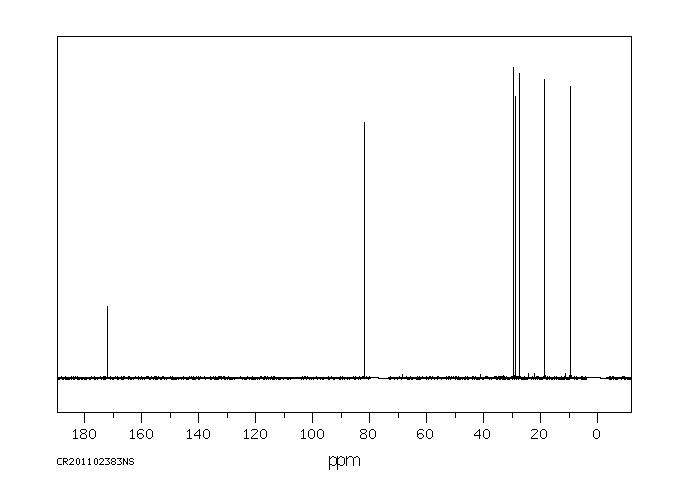
碳谱13CNMR
-
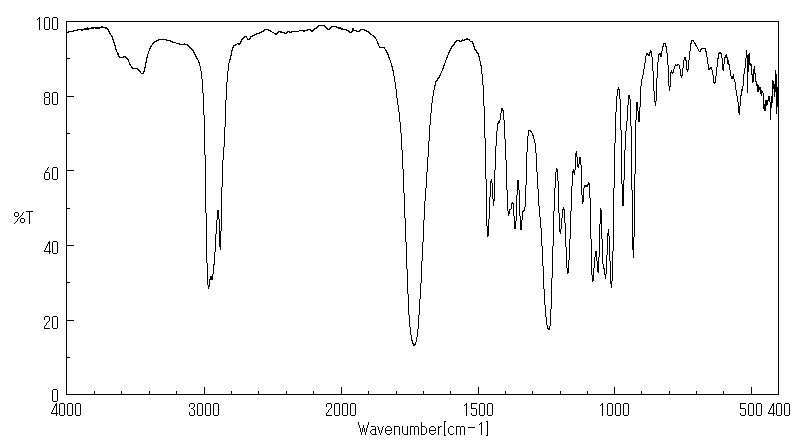
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(+)-(3R)-3-{[叔丁基(二甲基)硅基]氧基}二氢呋喃-2(3H)-酮
龙胆黄碱
龙胆酮胺
高良姜萜内酯
高柠檬酸-gamma-内酯
高普伐他汀内酯二-(叔-丁基二甲基硅烷基)醚
马桑内酯
顺式蒈醛酸内酯
顺式-3,5-二甲基二氢-2H-吡喃-2,6(3H)-二酮
顺式-1,3-环戊烷二甲酸酐
顺式-1,3-环己烷二甲酸酐
阿拉伯酸,2-氨基-2,3,5-三脱氧-3-甲基-,γ-内酯(9CI)
酸,(1S,3R,4R,5R)-3,4-二羟基-7-羰基-6-氧杂二环[3.2.1]辛-1-基2,2,2-三氯乙基酯碳
辛伐他汀4'-甲基醚
辛伐他汀
软木三萜酮3,4-内酯
试剂Menthide
试剂6-Allyl-epsilon-caprolactone
表洛伐他汀
蜂毒
藻酸钠
薇甘菊内酯
葡醛内酯
葡庚糖酸内酯
葡庚糖酸內酯
莫那可林X
莫那可林L
莫那可林J
脱氢抗坏血酸
聚乌拉坦
聚(epsilon-己内酯-delta-戊内酯)
羟基马桑毒内酯
羟基蓍含蓍素
羟基己酸内酯与2,2-二甲基-1,3-丙二醇的聚合物
美伐他汀
绵毛马兜铃内酯
糖质酸-1,4-内酯
穿心莲内酯
科立内脂二醇
硫丹内酯
石蚕苷A
甲酰辛伐他汀
甲瓦龙酸内酯-D4
甲瓦龙酸内酯-D3
甲瓦龙酸内酯-1-13C
甲瓦龙酸内酯-1,2-13C2
甲瓦龙酸内酯
甲基丙烯酸甲瓦龙酸内酯
甲基[(1S,5R,6R)-3-氧代-2-氧杂双环[3.2.1]辛-6-基]乙酸酯
瑞舒伐他汀杂质113