triptamin ammonium ion | 65838-59-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:12
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:43.4
-
氢给体数:2
-
氢受体数:0
反应信息
-
作为反应物:描述:开联番木鳖苷 、 triptamin ammonium ion 在 His-tagged Rauvolfia serpentina strictosidine synthase 作用下, 生成 3-α(S)-strictosidine参考文献:名称:胡豆苷合酶双底物活性中的结合顺序和表观结合亲和力摘要:具有双底物活性的萝芙木蛇胡豆苷合酶 (RsSTR) 是单萜吲哚生物碱 (MIA) 生物合成途径的核心,因为它立体选择性地缩合...DOI:10.1080/07391102.2023.2193643
-
作为产物:描述:参考文献:名称:来自长春花细胞悬浮培养物的色氨酸脱羧酶:均质蛋白的纯化、分子和动力学数据。摘要:描述了从长春花 (TDC, EC:4.1.1.27) 中纯化色氨酸脱羧酶,使其具有明显的同质性。该酶代表一种分子量为 115 000±3 000 的可溶性蛋白质,由 2 个相同的亚基 54 000±1 000 组成。 pI 估计为 5.9,L-色氨酸的 Km 为 7.5×10 (-5) M.苯丙氨酸、酪氨酸和多巴未被来自长春花细胞的色氨酸脱羧酶脱羧。类似于来自猪肾的芳香族氨基酸脱羧酶,该酶似乎并不一定依赖于外源供应的磷酸吡哆醛,因为它似乎含有一定量的这种辅因子。发现细胞中 TDC 的平均百分比在生长培养基中为 0.002%,而当诱导吲哚生物碱生物合成时,该水平增加至 0.03%。DOI:10.1007/bf00017782
文献信息
-
Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode <i>N</i> -acetylserotonin deacetylase, which catalyzes conversion of <i>N</i> -acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants作者:Kyungjin Lee、Hyoung Yool Lee、Kyoungwhan BackDOI:10.1111/jpi.12460日期:2018.3screened 4 genes that were known as histone deacetylase (HDAC) genes, but encoded proteins targeted into chloroplasts or mitochondria rather than nuclei. Of 4 recombinant Escherichia coli strains expressing these genes, one E. coli strain expressing the rice HDAC10 gene was found to be capable of producing serotonin in response to treatment with NAS. The recombinant purified rice HDAC10 (OsHDAC10)在植物中,褪黑激素的产生受到严格的调节,这与其褪色素的前体的产生不同,后者是对衰老和病原体暴露等刺激产生高度诱导作用的。外源性5-羟色胺处理不会在植物中极大地诱导N-乙酰5-羟色胺(NAS)和褪黑激素的产生,这表明从5-羟色胺生物合成褪黑激素的途径中可能存在一个或多个调控基因。在此报告中,我们发现NAS在水稻幼苗中迅速大量转化为5-羟色胺,表明存在N-乙酰5-羟色胺脱乙酰基酶(ASDAC)。为了克隆假定的ASDAC基因,我们筛选了4个被称为组蛋白脱乙酰基酶(HDAC)的基因。)基因,但编码的蛋白质靶向叶绿体或线粒体而不是细胞核。在表达这些基因的4种重组大肠杆菌菌株中,发现一种表达水稻HDAC10基因的大肠杆菌菌株能够响应于NAS处理而产生5-羟色胺。重组纯化的水稻HDAC10(OsHDAC10)蛋白对NAS,N-乙酰酪胺(NAT),N具有ASDAC酶活性-乙酰色胺和褪黑激素,对于NAT具有
-
Sekiguchi Lesion Gene Encodes a Cytochrome P450 Monooxygenase That Catalyzes Conversion of Tryptamine to Serotonin in Rice作者:Tadashi Fujiwara、Sylvie Maisonneuve、Masayuki Isshiki、Masaharu Mizutani、Letian Chen、Hann Ling Wong、Tsutomu Kawasaki、Ko ShimamotoDOI:10.1074/jbc.m109.091371日期:2010.4protein exhibited tryptamine 5-hydroxylase enzyme activity and catalyzed the conversion of tryptamine to serotonin. This pathway is novel and has not been reported in mammals. Expression of was induced by the -acetylchitooligosaccharide (chitin) elicitor and by infection with , a causal agent for rice blast disease. Exogenously applied serotonin induced defense gene expression and cell death in rice suspension血清素是哺乳动物中众所周知的神经递质,在人类的各种心理功能中发挥着重要作用。在植物中,血清素生物合成途径及其功能尚不清楚。水稻sekiguchi lesion ()突变体积累色胺,色胺是血清素生物合成的候选底物。我们通过基于图位的克隆分离了该基因,发现它编码细胞色素 P450 单加氧酶家族中的 CYP71P1。重组SL蛋白表现出色胺5-羟化酶活性并催化色胺转化为血清素。该途径是新颖的,在哺乳动物中尚未有报道。的表达是由β-乙酰壳寡糖(几丁质)诱导子和稻瘟病病原体感染诱导的。外源应用血清素诱导水稻悬浮培养物中的防御基因表达和细胞死亡,并增加植物对稻瘟病感染的抵抗力。我们还发现,5-羟色胺诱导的防御基因表达是由 RacGTPase 途径和异源三聚体 G 蛋白的 Gα 亚基介导的。这些结果表明血清素在水稻先天免疫中发挥着重要作用。
-
The Structure of <i>Rauvolfia serpentina</i> Strictosidine Synthase Is a Novel Six-Bladed β-Propeller Fold in Plant Proteins作者:Xueyan Ma、Santosh Panjikar、Juergen Koepke、Elke Loris、Joachim StöckigtDOI:10.1105/tpc.105.038018日期:2006.4.3
Abstract The enzyme strictosidine synthase (STR1) from the Indian medicinal plant Rauvolfia serpentina is of primary importance for the biosynthetic pathway of the indole alkaloid ajmaline. Moreover, STR1 initiates all biosynthetic pathways leading to the entire monoterpenoid indole alkaloid family representing an enormous structural variety of ∼2000 compounds in higher plants. The crystal structures of STR1 in complex with its natural substrates tryptamine and secologanin provide structural understanding of the observed substrate preference and identify residues lining the active site surface that contact the substrates. STR1 catalyzes a Pictet-Spengler–type reaction and represents a novel six-bladed β-propeller fold in plant proteins. Structure-based sequence alignment revealed a common repetitive sequence motif (three hydrophobic residues are followed by a small residue and a hydrophilic residue), indicating a possible evolutionary relationship between STR1 and several sequence-unrelated six-bladed β-propeller structures. Structural analysis and site-directed mutagenesis experiments demonstrate the essential role of Glu-309 in catalysis. The data will aid in deciphering the details of the reaction mechanism of STR1 as well as other members of this enzyme family.
摘要 来自印度草药植物Rauvolfia serpentina的酶STrictosidine synthase (STR1) 对于吲哚生物碱ajmaline的生物合成途径至关重要。此外,STR1启动了所有导致整个单萜吲哚生物碱家族的生物合成途径,代表了植物中大约2000种化合物的巨大结构变化。STR1与其天然底物tryptamine和secologanin结合的晶体结构提供了对观察到的底物偏好的结构理解,并确定了接触底物的活性位点表面上的残基。STR1催化Pictet-Spengler型反应,并代表了植物蛋白中的一种新的六叶片β-螺旋桨折叠。基于结构的序列比对揭示了一个常见的重复序列模体(三个疏水残基后跟一个小残基和一个亲水残基),表明STR1和几个序列无关的六叶片β-螺旋桨结构之间可能存在进化关系。结构分析和位点定向突变实验表明Glu-309在催化中起着必不可少的作用。这些数据将有助于解密STR1以及这种酶家族的其他成员的反应机制的细节。
-
3D-Structure and function of strictosidine synthase – the key enzyme of monoterpenoid indole alkaloid biosynthesis作者:Joachim Stöckigt、Leif Barleben、Santosh Panjikar、Elke A. LorisDOI:10.1016/j.plaphy.2007.12.011日期:2008.3Strictosidine synthase (STR; EC 4.3.3.2) plays a key role in the biosynthesis of monoterpenoid indole alkaloids by catalyzing the Pictet-Spengler reaction between tryptamine and secologanin, leading exclusively to 3alpha-(S)-strictosidine. The structure of the native enzyme from the Indian medicinal plant Rauvolfia serpentina represents the first example of a six-bladed four-stranded beta-propellerSTrictosidine合酶(STR; EC 4.3.3.2)通过催化色胺和sECologanin之间的Pictet-Spengler反应,在单萜类吲哚生物碱的生物合成中起关键作用,仅导致3alpha-(S)-STrictosidine。来自印度药用植物蛇纹螺旋藻的天然酶的结构代表了来自植物界的六叶四链β-螺旋桨折叠的第一个例子。此外,酶-底物和酶-产物复合物的结构揭示了对合酶活性中心和机理的深入了解,突出了Glu309作为催化残基的重要性。本综述描述了R的3D结构和功能。蛇纹石速尿苷合酶,并提供了迄今为止在不同生物中进行的速尿苷合酶底物特异性研究的摘要。基于酶-产物复合物,本文继续描述了该酶的合理的,基于结构的重新设计,这提供了产生可用于产生N-类似物异育亨宾类型的生物碱文库的新的严格糖苷衍生物的机会。最后,介绍了功能性表达的芥子酸合酶的比对研究,并讨论了与序列和结构相关的β-螺旋桨折
-
Strictosidine: From alkaloid to enzyme to gene作者:Toni M. KutchanDOI:10.1016/s0031-9422(00)95128-8日期:1993.2biochemical characteristics of the biosynthetic enzyme, strictosidine synthase, a new approach to the study of monoterpenoid indole alkaloid biosynthesis was developed. Physiological studies of monoterpenoid indole alkaloid biosynthesis at the enzymic level in plants and plant cell cultures were performed followed by the analyses of these systems at the level of molecular genetics.
表征谱图
-
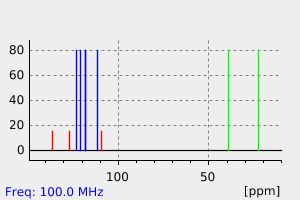
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息