N,N-二乙基-2-硝基苯胺 | 2216-17-3
中文名称
N,N-二乙基-2-硝基苯胺
中文别名
邻硝基二乙基苯胺;N,N-二乙基邻硝基苯胺;2-硝基-N,N-二乙基苯胺
英文名称
N,N-diethyl-2-nitrobenzenamine
英文别名
2-nitro-N,N-diethylaniline;N,N-Diethyl-o-nitroanilin;2-Diethylamino-1-nitro-benzol;2-Nitro-diaethylanilin;N,N-Diaethyl-2-nitro-anilin;N,N-diethyl-2-nitro-aniline;2-Nitro-N,N-diaethyl-anilin;N,N-Diethyl-2-nitroaniline
CAS
2216-17-3
化学式
C10H14N2O2
mdl
MFCD01120260
分子量
194.233
InChiKey
BUBRUAQVAPTGHV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:49.1
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,N-二乙基-1,2-苯二胺 N1,N1-diethylbenzene-1,2-diamine 19056-34-9 C10H16N2 164.25
反应信息
-
作为反应物:描述:N,N-二乙基-2-硝基苯胺 在 palladium 10% on activated carbon 、 氢气 、 三乙胺 作用下, 以 甲醇 、 乙腈 为溶剂, 反应 10.0h, 生成 N1-(1,3-dimethylimidazolidin-2-ylidene)-N2,N2-diethylbenzene-1,2-diamine参考文献:名称:氯化锌与脂肪族和芳香族胍杂合配体的配合物及其在 d,l-丙交酯开环聚合中的活性摘要:报道了新的杂化胍配体 DMEGdmap、DMEGdeae、TMGdmab、DMEGdmab、TMGdeab 和 DMEGdeab 的合成。这些配体与氯化锌结合,得到的六种新配合物通过 X 射线晶体学和核磁共振光谱进行了结构表征。从杂化胍配体 TMGdmae、DMEGdmae、TMGdeae、TMGdmap、TMGdeap 和 TEGdeap 中获得了另外六个新的氯化锌配合物。十二个配合物中的每一个都具有四面体配位几何。使用密度泛函理论评估胍和胺供体之间的供体情况。这些配合物在工业未升华丙交酯的熔融聚合中显示出强大的活性。对于选定的复合物,进行了动力学聚合实验;这些表现出一阶行为。DOI:10.1002/ejic.201600870
-
作为产物:描述:硝基氯苯 、 二乙胺 在 copper(l) iodide 、 乌洛托品 、 caesium carbonate 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 20.0h, 生成 N,N-二乙基-2-硝基苯胺参考文献:名称:新型3-(二氟甲基)-1-甲基-1H-吡唑-4-羧酸酰胺的合成,抗真菌活性和结构活性关系摘要:合成了一系列新型的3-(二氟甲基)-1-甲基-1H-吡唑-4-羧酸酰胺,并通过体外菌丝体生长抑制试验测试了它们对七种植物病原真菌的活性。他们大多数表现出中等至出色的活动。其中N-(2-(5-溴-1H-吲唑-1-基)苯基)-3-(二氟甲基)-1-甲基-1H-吡唑-4-羧酰胺(9m)具有较高的抗真菌活性七种植物病原性真菌要比Boscalid好。用Topomer CoMFA建立了化合物的三维定量构效关系模型。在分子对接中,9m的羰基氧原子可与SDH上的TYR58和TRP173的羟基形成氢键。DOI:10.3390/molecules20058395
文献信息
-
1,2-Disubstituted Benzimidazoles by the Iron Catalyzed Cross-Dehydrogenative Coupling of Isomeric <i>o</i>-Phenylenediamine Substrates作者:Pawan Thapa、Philip M. Palacios、Tam Tran、Brad S. Pierce、Frank W. FossDOI:10.1021/acs.joc.9b02714日期:2020.2.21Benzimidazoles are common in nature, medicines, and materials. Numerous strategies for preparing 2-arylbenzimidazoles exist. In this work, 1,2-disubstituted benzimidazoles were prepared from various mono- and disubstituted ortho-phenylenediamines (OPD) by iron-catalyzed oxidative coupling. Specifically, O2 and FeCl3·6H2O catalyzed the cross-dehydrogenative coupling and aromatization of diarylmethyl苯并咪唑在自然界,药物和材料中很常见。存在制备2-芳基苯并咪唑的许多策略。在这项工作中,通过铁催化的氧化偶联,由各种单取代和二取代的邻苯二胺(OPD)制备1,2-二取代的苯并咪唑。具体而言,O 2和FeCl 3·6H 2 O催化二芳基甲基和二烷基苯并咪唑前体的交叉脱氢偶联和芳构化。N,N′-二取代-OPD底物比其N,N-二取代的异构体具有更高的反应活性,这似乎与其相对于与Fe3 +络合和电荷转移的倾向有关。该反应还将N-单取代的OPD底物转化为2-取代的苯并咪唑。然而,贫电子底物通过分子间亚氨基转移产生1,2-二取代的苯并咪唑。动能 试剂和光谱学(紫外可见和EPR)研究表明,这种机制涉及金属-底物的络合,电荷转移和有氧转化,涉及高价Fe(IV)中间体。总的来说,证明了比较可持续和有效合成1,2-二取代苯并咪唑的比较策略。
-
一系列四芳基螺环化合物及其制备方法与应 用申请人:天津师范大学公开号:CN109574822B公开(公告)日:2021-12-21
-
Synthesis of benzimidazoles via iridium-catalyzed acceptorless dehydrogenative coupling
-
Optimisation of 2-(N-phenyl carboxamide) triazolopyrimidine antimalarials with moderate to slow acting erythrocytic stage activity作者:Brodie L. Bailey、William Nguyen、Anna Ngo、Christopher D. Goodman、Maria R. Gancheva、Paola Favuzza、Laura M. Sanz、Francisco-Javier Gamo、Kym N. Lowes、Geoffrey I. McFadden、Danny W. Wilson、Benoît Laleu、Stephen Brand、Paul F. Jackson、Alan F. Cowman、Brad E. SleebsDOI:10.1016/j.bioorg.2021.105244日期:2021.10the Janssen Jumpstarter library against the asexual stages of the P. falciparum parasite. Here we describe the structure activity relationship of the identified class and the optimisation of asexual stage activity while maintaining selectivity against the human HepG2 cell line. The most potent analogues from this study were shown to exhibit equipotent activity against P. falciparum multidrug resistant疟疾是由从该属的寄生虫毁灭性的寄生虫病疟原虫。据报道,所有临床上可用的抗疟药都存在治疗耐药性,威胁到我们控制疾病的能力,因此持续需要开发新型抗疟药。为了实现这一目标,我们从 Janssen Jumpstarter 库的高通量筛选中针对恶性疟原虫的无性阶段鉴定了 2-(N-苯基甲酰胺)三唑并嘧啶类寄生虫。在这里,我们描述了已识别类别的结构活性关系和无性阶段活性的优化,同时保持对人 HepG2 细胞系的选择性。本研究中最有效的类似物显示出对恶性疟原虫多药耐药菌株和诺氏疟原虫无性寄生虫的等效活性。无性阶段表型研究确定三唑并嘧啶类在滋养体阶段捕获寄生虫,但这些寄生虫很可能在第二个无性周期之前仍然具有代谢活性,因此具有中度至缓慢的作用开始。在体外观察到中心羧酰胺的非 NADPH 依赖性降解和低水溶性ADME 分析。一个重大挑战仍然是纠正这些缺陷,以进一步推进 2-( N-苯基甲酰胺) 三唑并嘧啶支架
-
<i>tert</i>-Amino Effect-Promoted Rearrangement of Aryl Isothiocyanate: A Versatile Approach to Benzimidazothiazepines and Benzimidazothioethers作者:Xinyu Geng、Siyuan Liu、Wenyao Wang、Jingping Qu、Baomin WangDOI:10.1021/acs.joc.0c01806日期:2020.10.2A general and practical approach to benzimidazothiazepine and benzimidazothioether derivatives via an intramolecular nucleophilic addition/ring expansion rearrangement of aryl isothiocyanates promoted by the tert-amino effect has been developed. This reaction is catalyzed by low-cost camphorsulfonic acid and tolerates a broad substrate scope with complete atom economy. Structurally intriguing benzimidazothiazepine
表征谱图
-
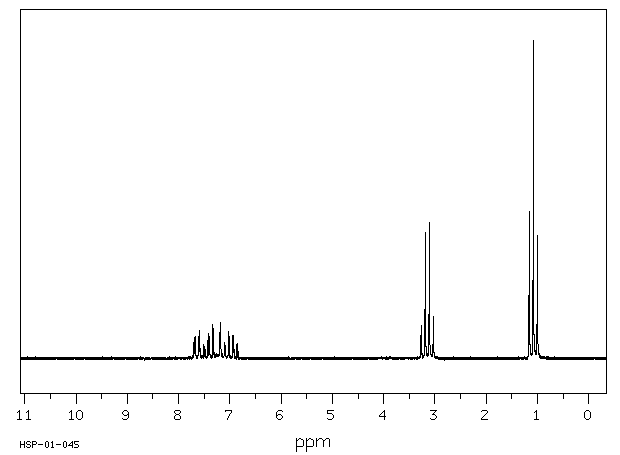
氢谱1HNMR
-
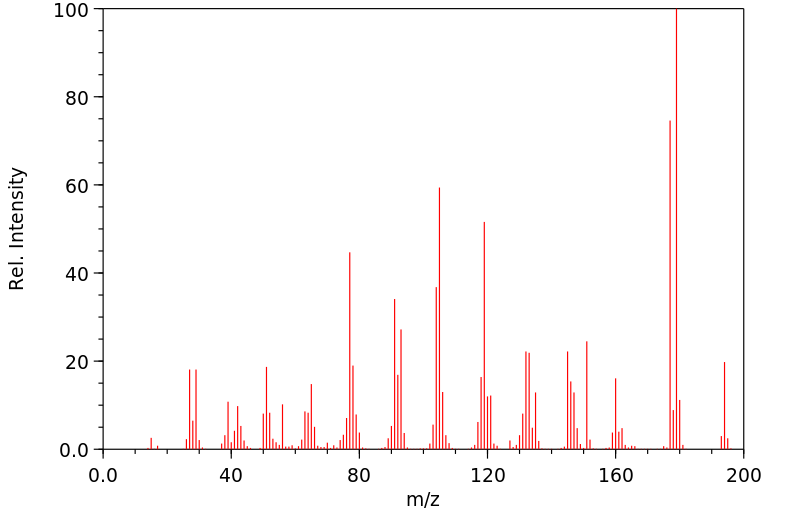
质谱MS
-
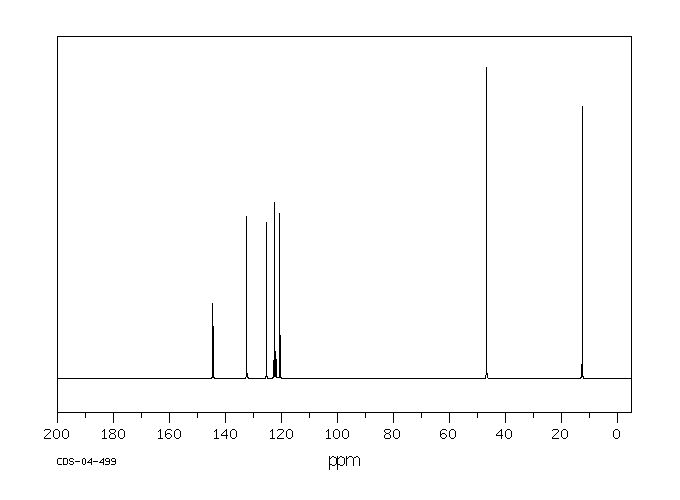
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫