N-乙基丙酰胺 | 5129-72-6
中文名称
N-乙基丙酰胺
中文别名
氮丙乙基胺
英文名称
N-ethylpropionamide
英文别名
N-propionyl-ethyl amine;N-ethylpropanamide
CAS
5129-72-6
化学式
C5H11NO
mdl
MFCD00059388
分子量
101.148
InChiKey
ABMDIECEEGFXNC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:116 °C / 25mmHg
-
密度:0,91 g/cm3
-
保留指数:950
-
稳定性/保质期:
在常温常压下,该物质保持稳定。
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
海关编码:2924199090
-
储存条件:常温、避光、阴凉干燥处,密封保存。
SDS
N-乙基丙酰胺
模块 1. 化学品
产品名称: N-Ethylpropionamide
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): N-乙基丙酰胺
百分比: >99.0%(GC)
CAS编码: 5129-72-6
俗名: N-Propionylethylamine
分子式: C5H11NO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
N-乙基丙酰胺
模块 5. 消防措施
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 116 °C/3.3kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.91
溶解度:
[水] 无资料
[其他溶剂] 无资料
N-乙基丙酰胺
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
N-乙基丙酰胺
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: N-Ethylpropionamide
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): N-乙基丙酰胺
百分比: >99.0%(GC)
CAS编码: 5129-72-6
俗名: N-Propionylethylamine
分子式: C5H11NO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
N-乙基丙酰胺
模块 5. 消防措施
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 116 °C/3.3kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.91
溶解度:
[水] 无资料
[其他溶剂] 无资料
N-乙基丙酰胺
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
N-乙基丙酰胺
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-chloro-N-ethylpropionamide 82890-91-3 C5H10ClNO 135.594
反应信息
-
作为反应物:参考文献:名称:Hydroformylation of allyl alcohol摘要:公开号:EP0151515B1
-
作为产物:参考文献:名称:微波辅助高效一步合成酮和乙二氧肟酸,由酮高效催化一步合成酮类酰胺,由(2-羟芳基)酮催化苯并恶唑摘要:描述了一种有效的一步法,该方法通过使用硫酸作为催化剂,通过乙酰氧肟酸反应,直接从酮中直接合成酰胺,从(2-羟基芳基)酮中合成苯并恶唑。DOI:10.1016/j.tetlet.2011.09.019
-
作为试剂:参考文献:名称:DE726290摘要:公开号:
文献信息
-
TMSOTf-mediated approach to 1,3-oxazin-2-one skeleton through one-pot successive reduction-[4 + 2] cyclization process of imides with ynamides作者:Chen-Chen Zhang、Zhi-Peng Huo、Mei-Lin Tang、Yong-Xi Liang、Xun SunDOI:10.1016/j.tetlet.2021.152946日期:2021.3A one-pot approach to access functionalized 1,3-oxazin-2-one skeleton has been developed through successive reduction and subsequent [4 + 2] cyclization process of N-Boc lactams with ynamides by TMSOTf. As a result, a number of five to seven membered ring fused bicyclic [1,2-c][1], [3]oxazin-1-ones 12a-m and tricyclic derivatives 13a-f were obtained in moderate to excellent yields with excellent regioselectivities
-
Scope and Mechanism of a True Organocatalytic Beckmann Rearrangement with a Boronic Acid/Perfluoropinacol System under Ambient Conditions作者:Xiaobin Mo、Timothy D. R. Morgan、Hwee Ting Ang、Dennis G. HallDOI:10.1021/jacs.8b01618日期:2018.4.18enylboronic acid were identified as efficient catalysts for the direct and chemoselective activation of oxime N-OH bonds in the Beckmann rearrangement. This classical organic reaction provides a unique approach to prepare functionalized amide products that may be difficult to access using traditional amide coupling between carboxylic acids and amines. Using only 5 mol % of boronic acid catalyst and羟基官能团的催化活化对于药物和商品化学品的生产具有重要意义。在这里,2-烷氧基羰基-和 2-苯氧基羰基-苯基硼酸被确定为在贝克曼重排中直接和化学选择性活化肟 N-OH 键的有效催化剂。这种经典的有机反应提供了一种独特的方法来制备官能化的酰胺产物,使用传统的羧酸和胺之间的酰胺偶联可能难以获得这些产物。在极性溶剂混合物中仅使用 5 mol% 的硼酸催化剂和全氟频哪醇作为添加剂,操作简单的方案具有条件温和、底物范围广和官能团耐受性高的特点。种类繁多的二芳基、芳基-烷基、杂芳基-烷基、和二烷基肟在环境条件下反应以提供高产率的酰胺产物。游离醇、酰胺、羧酸酯和许多其他官能团与反应条件相容。对催化循环的研究揭示了一种新型的硼诱导的肟酯交换,它提供了一种酰基肟中间体,参与了完全催化的非自蔓延贝克曼重排机制。酰基肟中间体独立制备并经受反应条件。它被发现是自给自足的;它反应迅速,单分子,不需要游离肟。一系列对照实验和
-
Eine neue Methode zur Reduktion eines Amids zum Amin mit der gleichen Anzahl von Kohlenstoffatomen作者:A. Uffer、E. SchlittlerDOI:10.1002/hlca.19480310526日期:——An Hand von 9 Beispielen wird gezeigt, dass sich Säureamide im allgemeinen leicht mit Lithium-Aluminium-Hydrid zu den entspre-chenden Aminen reduzieren lassen.9个实施例表明,通常可以用氢化锂铝将酰胺容易地还原为相应的胺。
-
An Electrochemical Beckmann Rearrangement: Traditional Reaction via Modern Radical Mechanism作者:Li Tang、Zhi‐Lv Wang、Yan‐Hong He、Zhi GuanDOI:10.1002/cssc.202001553日期:2020.9.18electrochemical Beckmann rearrangement, i. e. the direct electrolysis of ketoximes to amides, is presented for the first time. Using a constant current as the driving force, the reaction can be easily carried out under neutral conditions at room temperature. Based on a series of mechanistic studies, a novel radical Beckmann rearrangement mechanism is proposed. This electrochemical Beckmann rearrangement does
-
Autoxidation of N-alkylamides. Part I. N-Acylamides as oxidation products作者:M. V. Lock、B. F. SagarDOI:10.1039/j29660000690日期:——Products of the thermal and photosensitised autoxidation of N-alkyl- and NN-dialkyl-amides have been identified. N-n-Alkylamides yield principally N-acylamides, primary amides, and N-formylamides, as a result of initial abstraction of a hydrogen atom from the carbon adjacent to nitrogen. Formation of N-formylamides, and of N-acylamides from N-s-alkylamides, involves C(1)–C(2) bond scission in an N-alkyl已经鉴定了N-烷基-和NN-二烷基酰胺的热和光敏自氧化的产物。Ñ -n烷基酰胺得到主要Ñ -acylamides,伯酰胺,和Ñ -formylamides,如从与氮相邻碳氢原子的初始抽象的结果。的形成Ñ -formylamides,以及Ñ从-acylamides Ñ -s-三烷基酰胺,涉及Ç (1) -C (2)键的断裂中ñ -烷基。NN的氧化-二烷基酰胺遵循相似的模式。给出了89种酰胺的气相色谱-液相色谱保留数据。
表征谱图
-
氢谱1HNMR
-
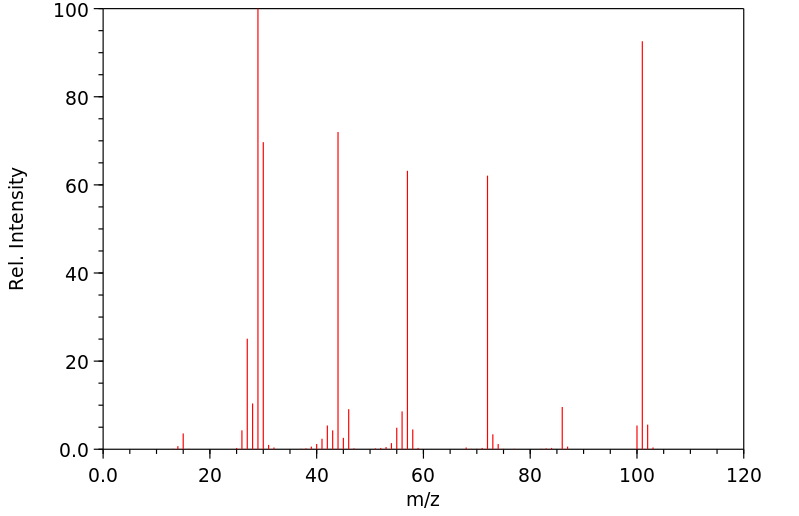
质谱MS
-
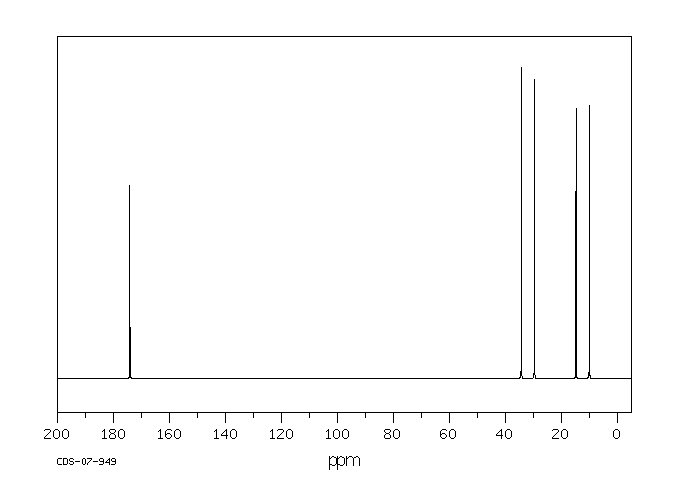
碳谱13CNMR
-
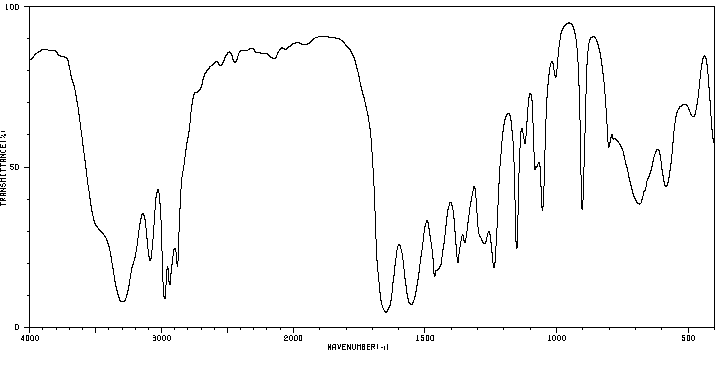
红外IR
-
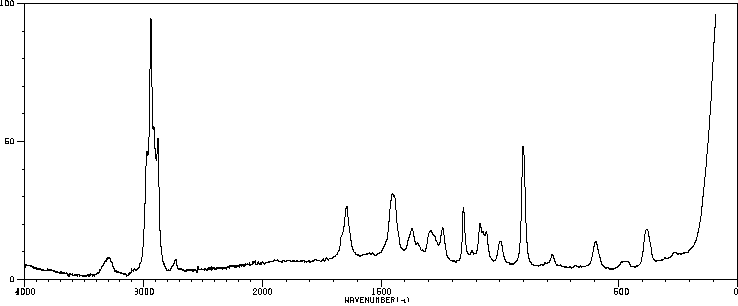
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸