草酸二苯酯 | 3155-16-6
中文名称
草酸二苯酯
中文别名
——
英文名称
diphenyl oxalate
英文别名
Diphenyl-oxalat;Oxalsaeure-diphenylester;DPO;Diphenyl ethanedioate;oxalic acid diphenyl ester
CAS
3155-16-6
化学式
C14H10O4
mdl
MFCD00059682
分子量
242.231
InChiKey
ULOZDEVJRTYKFE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:136°C
-
沸点:336.7±15.0 °C(Predicted)
-
密度:1.257±0.06 g/cm3(Predicted)
-
稳定性/保质期:
遵照规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:18
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
安全说明:S36/37/39
-
危险类别码:R22,R36/37/38
-
海关编码:2917119000
-
包装等级:III
-
危险类别:9
-
危险性防范说明:P260,P264,P273,P301+P312,P305+P351+P338,P314
-
危险品运输编号:3077
-
危险性描述:H302,H319,H372,H410
-
储存条件:存放于阴凉干燥处。
SDS
Diphenyl Oxalate Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Diphenyl Oxalate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Harmful if swallowed
Precautionary statements:
[Prevention] Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
[Response]
Rinse mouth.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Diphenyl Oxalate
Percent: >98.0%(GC)
CAS Number: 3155-16-6
Synonyms: Oxalic Acid Diphenyl Ester
Chemical Formula: C14H10O4
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Diphenyl Oxalate
Section 4. FIRST AID MEASURES
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: Crystal- Powder
Colour: White - Pale reddish yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:136°C
Boiling point/range: No data available
No data available
Flash point:
Diphenyl Oxalate
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] Insoluble
[Other solvents]
Soluble: Acetone
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: orl-mus LD50:1 g/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
RO2880000
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Diphenyl Oxalate
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Diphenyl Oxalate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Harmful if swallowed
Precautionary statements:
[Prevention] Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
[Response]
Rinse mouth.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Diphenyl Oxalate
Percent: >98.0%(GC)
CAS Number: 3155-16-6
Synonyms: Oxalic Acid Diphenyl Ester
Chemical Formula: C14H10O4
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Diphenyl Oxalate
Section 4. FIRST AID MEASURES
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: Crystal- Powder
Colour: White - Pale reddish yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:136°C
Boiling point/range: No data available
No data available
Flash point:
Diphenyl Oxalate
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] Insoluble
[Other solvents]
Soluble: Acetone
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: orl-mus LD50:1 g/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
RO2880000
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Diphenyl Oxalate
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
概述
碳酸二苯酯(DPC)是一种重要的有机化合物,是非光气法制备聚碳酸酯的基本原料。近年来,由于聚碳酸酯具有优异的机械、光学和电子性能,其应用领域日益广泛,从而使得DPC的研究与开发逐渐成为热点。
目前,非光气法合成DPC的主要方法包括酯交换法和氧化羰基化法。具体来说,苯酚与草酸二甲酯(DMO)通过酯交换反应生成草酸二苯酯(DPO),然后再进行脱羰基反应生成DPC。这种方法中,副产物甲醇及二氧化碳容易从反应体系中分离出来,避免了采用碳酸二甲酯和苯酚酯交换合成DPC过程中,副产物甲醇与原料碳酸二甲酯形成共沸物的问题,从而简化了后续的分离步骤。
此外,此方法为常压操作,条件温和,使用的催化剂成本较低且容易获得。因此,这是一种极具发展潜力的工艺路线。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— phenyl oxalyl chloride 51719-70-1 C8H5ClO3 184.579 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— di-1-naphthyl oxalate 94644-74-3 C22H14O4 342.351 双(4-硝基苯基)草酸盐 Bis-<4-nitro-phenyl>-oxalat 5070-15-5 C14H8N2O8 332.226 —— phenyl oxalyl chloride 51719-70-1 C8H5ClO3 184.579 —— bis(3-nitrophenyl) oxalate 93716-52-0 C14H8N2O8 332.226
反应信息
-
作为反应物:参考文献:名称:PROCESS FOR PREPARING DIARYL OXALATE摘要:本发明公开了一种制备二芳基草酸酯的方法,其中包括在四(芳氧基)钛存在下,将二烷基草酸酯和/或烷基芳基草酸酯与芳基醇进行酯交换反应的步骤。其中,四(芳氧基)钛以四芳氧基钛和过量的芳基醇反应,去除副产生的烷基醇后,作为四(芳氧基)钛的芳基醇溶液加入到酯交换反应的反应体系中。公开号:US20120296063A1
-
作为产物:参考文献:名称:Process for the preparation of aromatic polycarbonate摘要:一种制备芳香族聚碳酸酯的方法,通过芳香族碳酸二酯和芳香族二羟基化合物之间的酯交换反应,其中芳香族碳酸二酯是通过下列通式(1)所代表的芳香族草酸二酯的脱羰基反应获得的:其中两个Ar是具有6至14个碳原子的相同或不同的芳香族碳氢基团,并且具有5ppm或更低的可水解卤素含量。根据本发明,通过使用通过芳香族草酸二酯的脱羰基反应获得的芳香族碳酸二酯,并控制芳香族碳酸二酯中可水解卤素的含量小于预定值,可以轻松地生产具有高分子量和优异颜色的芳香族聚碳酸酯,而不会损害用于生产芳香族聚碳酸酯的酯交换反应的反应性。公开号:US06265524B1
-
作为试剂:描述:3-碘-2-甲基苯甲酸甲酯 在 N-溴代丁二酰亚胺(NBS) 、 copper(l) iodide 、 四(三苯基膦)钯 、 草酸二苯酯 、 palladium 10% on activated carbon 、 氢气 、 三乙胺 、 cesium fluoride 作用下, 以 N,N-二甲基甲酰胺 、 苯 为溶剂, 生成 C15H16N2O3参考文献:名称:来那度胺和泊马利度胺的等规类似物:合成与生物活性摘要:制备了一系列免疫调节药物来那度胺(1)和pomalidomide(2)的类似物,其中的氨基被各种等排物取代,并测定了其免疫调节活性和针对癌细胞系的活性。4-甲基和4-氯类似物4和15分别在LPS刺激的hPBMC中显示出对肿瘤坏死因子-α(TNF-α)的有效抑制作用,在人T细胞共刺激测定中对IL-2的有效刺激作用,以及对Namalwa淋巴瘤细胞系的抗增殖活性。这两种类似物在大鼠中均显示出口服生物利用度。DOI:10.1016/j.bmcl.2012.10.071
文献信息
-
Catalyst for decarbonylation reaction申请人:Ube Industries, Ltd.公开号:US05892091A1公开(公告)日:1999-04-06A catalyst composed of an organic phosphorus compound having a trivalent or pentavalent phosphorus atom and at least one carbon-phosphorus bonding or a combination of the organic phosphorus compound and a halogen atom-containing compound is effective for decarbonylation, that is, for releasing carbon monoxide from a compound containing a moiety of --CO--CO--O-- in its molecular structure.
-
[EN] NOVEL HYDROGEN PEROXIDE-ACTIVABLE, ANTI-OXIDANT COMPOUNDS AND METHODS USING SAME<br/>[FR] NOUVEAUX COMPOSÉS ANTI-OXYDANTS ACTIVABLES PAR PEROXYDE D'HYDROGÈNE, COMPOSÉS ANTI-OXYDANTS ET PROCÉDÉS LES UTILISANT申请人:BETH ISRAEL HOSPITAL公开号:WO2016073357A1公开(公告)日:2016-05-12The present invention includes 4-(hydroxymethyl)phenylboronic esters, which react with hydrogen peroxide to form 4-hydroxybenzyl alcohol, which is an anti-inflammatory and/or anti-oxidant compound. In certain embodiments, the compositions of the invention may be used to treat or prevent oxidative stress and/or inflammation, including ischemic disease.
-
HYBRID ANTICANCER PRODRUG SIMULTANEOUSLY PRODUCING CINNAMALDEHYDE AND QUINONE METHIDE AND METHOD FOR PREPARING SAME申请人:INDUSTRIAL COOPERATION FOUNDATION CHONBUK NATIONAL UNIVERSITY公开号:US20170014518A1公开(公告)日:2017-01-19The present invention relates to a hybrid anticancer prodrug simultaneously producing cinnamaldehyde and quinone methide. The hybrid anticancer prodrug according to the present invention sequentially releases quinone methide and cinnamaldehyde by H 2 O 2 and acidic pH, and thus alkylates antioxidant GSH through the release of quinone methide, thereby inhibiting an antioxidative system and increasing oxidation stress, and generates and accumulates reactive oxygen species (ROS) through the release of cinnamaldehyde, thereby promoting apoptosis, and thus the hybrid anticancer prodrug according to the present invention can be favorably used as an anticancer drug by creating a synergetic anticancer effect through double stimulus-response and sequential treatment action in a cancer cell-specific manner.
-
Method of preparation of oxalic acid esters and amides申请人:Enichem Anic S.p.A.公开号:US04981963A1公开(公告)日:1991-01-01A new process is described for the preparation of oxalic acid esters and amides of general formula (I) ##STR1## wherein Z designates an --OR or --NR.sup.1 R.sup.2 group, wherein R represents substituted or unsubstituted alkyl, alkenyl, cycloalkyl, aryl, or aryl-alkyl, R.sup.1 is hydrogen or substituted or unsubstituted alkyl, alkenyl, cycloalkyl, aryl, or aryl-alkyl, R.sup.2 represents substituted or unsubstituted alkyl, alkenyl, cycloalkyl, aryl, or aryl-alkyl, or R.sup.1 and R.sup.2 taken together with the adjacent nitrogen atom represent a saturated 5-, 6-, 7-, or 8-membered heterocyclic ring, which may contain an additional heteroatom selected from --O--, --S--, and --N(H, Alkyl)--, and optionally bear one or more alkyl or alkenyl substituents, and Z.sup.1 designates an --OR or --NR.sup.1 R.sup.2 group, wherein R, R.sup.1, and R.sup.2 are as defined before, or a group --NHCOCH.sub.3, which comprises the base-catalysed reaction of diacetyloxamide with an alcohol ROH or/and an amine HNR.sup.1 R.sup.2. The compounds of formula (I) have many industrial utilities, mainly as intermediates and stabilizers in the polymer field.描述了一种制备一般式(I)的草酸酯和酰胺的新工艺##STR1##其中Z代表--OR或--NR.sup.1 R.sup.2基团,其中R代表取代或未取代的烷基、烯基、环烷基、芳基或芳基-烷基,R.sup.1为氢或取代或未取代的烷基、烯基、环烷基、芳基或芳基-烷基,R.sup.2代表取代或未取代的烷基、烯基、环烷基、芳基或芳基-烷基,或R.sup.1和R.sup.2与相邻氮原子一起代表饱和的5、6、7或8元杂环,该环可能含有另外选择自--O--、--S--和--N(H,烷基)--的杂原子,并且可选地带有一个或多个烷基或烯基取代基,Z.sup.1代表--OR或--NR.sup.1 R.sup.2基团,其中R、R.sup.1和R.sup.2如前所定义,或一个--NHCOCH.sub.3基团,它包括二乙酰氧胺与醇ROH和/或胺HNR.sup.1 R.sup.2的碱催化反应。一般式(I)的化合物在聚合物领域中具有许多工业用途,主要用作中间体和稳定剂。
-
[EN] N-OXIDES OF 4,5-EPOXY-MORPHINANIUM ANALOGS<br/>[FR] N-OXYDES D'ANALOGUES 4,5-ÉPOXY-MORPHINANIUM申请人:PROGENICS PHARM INC公开号:WO2009067275A1公开(公告)日:2009-05-28Novel N-oxides of 4,5-epoxy-morphinanIum analogs are disclosed. Pharmaceutical compositions containing the N-oxides of 4,5-epoxy-morphinanium analogs and methods of their pharmaceutical uses are also disclosed. The compounds disclosed are useful, inter alia, as modulators of opioid receptors.
表征谱图
-
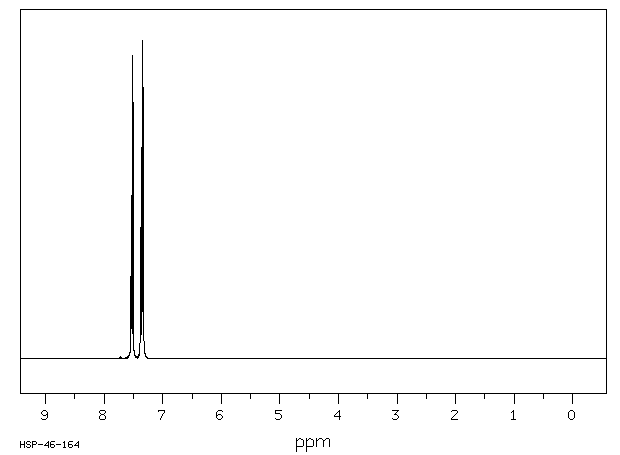
氢谱1HNMR
-
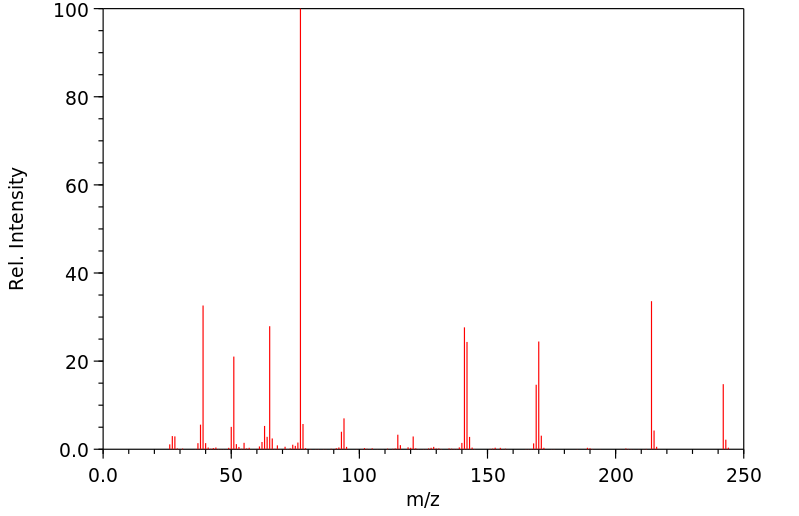
质谱MS
-
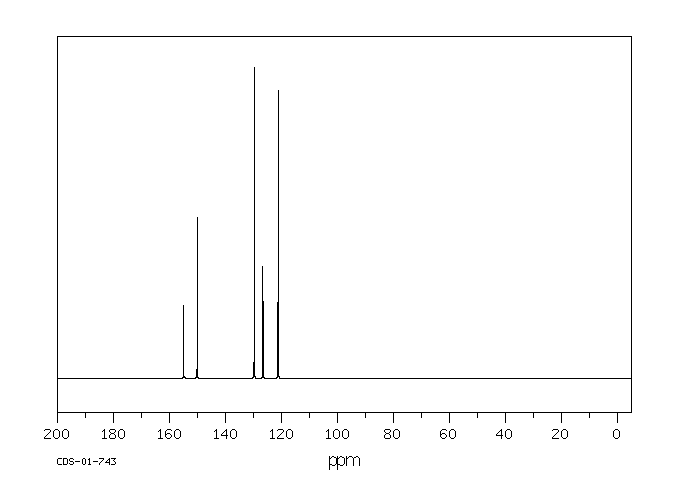
碳谱13CNMR
-
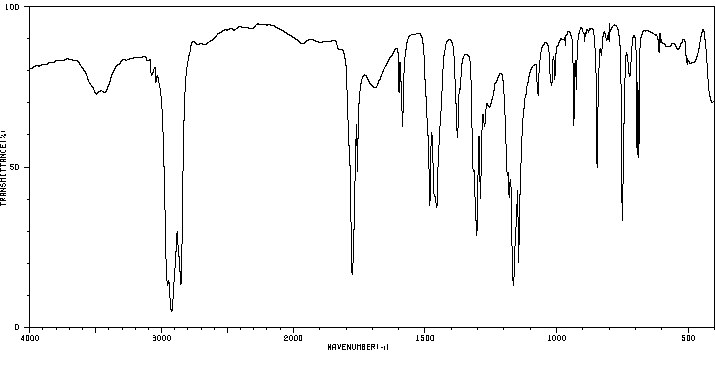
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫