4-Hydroxy-3-<3-furyl-(2)-acryloyl>-cumarin | 57340-06-4
中文名称
——
中文别名
——
英文名称
4-Hydroxy-3-<3-furyl-(2)-acryloyl>-cumarin
英文别名
1-(4-hydroxy-2-oxo-2H-chromen-3-yl)-3-(furan-2-yl)-2-propen-1-one;3-(3-furan-2-yl-acryloyl)-4-hydroxy-chromen-2-one
CAS
57340-06-4
化学式
C16H10O5
mdl
——
分子量
282.252
InChiKey
IXEQLIKVEUBGKR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.99
-
重原子数:21.0
-
可旋转键数:3.0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:80.65
-
氢给体数:1.0
-
氢受体数:5.0
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-乙酰基-2-羟基苯并吡喃-4-酮 3-acetyl-4-hydroxychromen-2-one 2555-37-5 C11H8O4 204.182
反应信息
-
作为反应物:参考文献:名称:Mustafa,A. et al., Justus Liebigs Annalen der Chemie, 1965, vol. 684, p. 194 - 200摘要:DOI:
-
作为产物:描述:参考文献:名称:Design, synthesis and biological evaluation of some novel 3-cinnamoyl-4-hydroxy-2H-chromen-2-ones as antimalarial agents摘要:A novel series of 3-cinnamoyl-4-hydroxy-2H-chromen-2-ones were designed, synthesized and screened for antiplasmodial activity. Eleven compounds of the series exhibited micromolar potency against chloroquine sensitive and chloroquine resistant strains. The most potent compound 4-hydroxy-3-(3-(4-nitrophenyl)acryloyl)-2H-chromen-2-one showed inhibitory potency (IC50) of 3.1 and 4 mu g/ml against chloroquine sensitive and chloroquine resistant strains, respectively. A structure activity relationship study was performed by correlating the effect of substituents with the antimalarial activity of the title compounds. The novel 3-cinnamoyl-4-hydroxy-2H-chromen-2-ones reported here should be good lead for further development of antimalarial agents that can overcome resistance.DOI:10.1007/s00044-011-9694-1
文献信息
-
Synthesis, anticoagulant and PIVKA-II induced by new 4-hydroxycoumarin derivatives作者:Omaima M. Abdelhafez、Kamelia M. Amin、Rasha Z. Batran、Timothy J. Maher、Somaia A. Nada、Shalini SethumadhavanDOI:10.1016/j.bmc.2010.04.009日期:2010.5The action of the coumarin-type drugs and related compounds is reviewed to their VKOR antagonistic effects. In our study, twenty 3-pyridinyl, pyrimidinyl and pyrazolyl-4-hydroxycoumarin derivatives were synthesized. A comparative in vivo (CT, PT determination) and in vitro ( measurement of PIVKA-II levels) anticoagulant study with respect to warfarin showed that the synthesized compounds have different anticoagulant activities, the most prospective compounds were the 3-pyrazolyl-4-hydroxycoumarin derivatives. (C) 2010 Elsevier Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
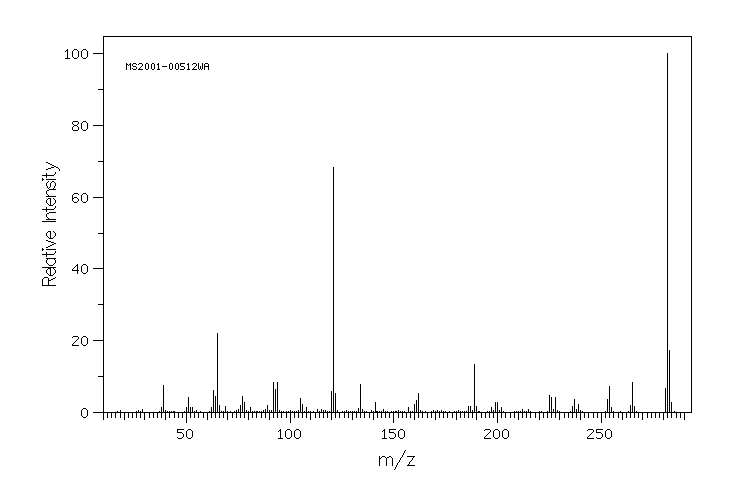
质谱MS
-
碳谱13CNMR
-
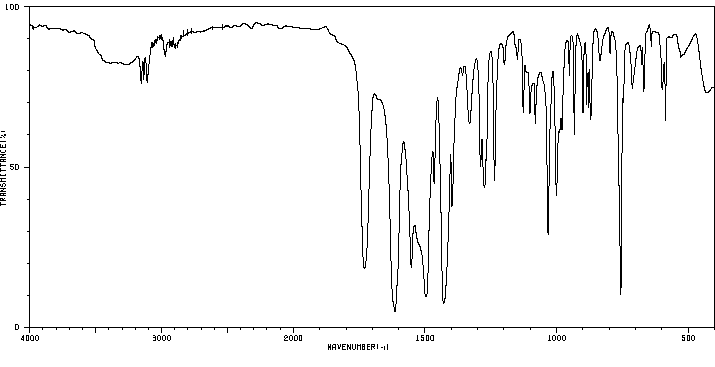
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄皮香豆精
黄木亭
黄曲霉素P2
黄曲霉素P1
黄曲霉素G2-13C17-同位素
黄曲霉素G2
黄曲霉素G1-13C17-同位素
黄曲霉素B2-13C17-同位素
黄曲霉素B1-13C17-同位素
黄曲霉素B1 8,9-环氧化物
黄曲霉素 G1
黄曲霉毒醇Ⅱ
黄曲霉毒醇M1
黄曲霉毒醇A
黄曲霉毒素M2
黄曲霉毒素M1-(O-羧甲基)肟
黄曲霉毒素G2a
黄曲霉毒素G19,10-环氧化物
黄曲霉毒素B2
黄曲霉毒素B1二氯化物
黄曲霉毒素B1-8,9-二氯化物
黄曲霉毒素B1-(O-羧甲基)肟
黄曲霉毒素 Q1
黄曲霉毒素 M1
黄曲霉毒素 B2
黄曲霉毒素 B1
黄曲霉毒素
香豆霉素
香豆素6H
香豆素545T
香豆素545
香豆素525
香豆素343甲酯
香豆素338
香豆素314T
香豆素175
香豆素152
香豆素106
香豆素-D4
香豆素-6-磺酰氯
香豆素-6-甲醛
香豆素-5-氧丁酸
香豆素-4-乙酸
香豆素-3腈
香豆素-35
香豆素-3-羧酸酸酐
香豆素-3-羧酸琥珀酰亚胺酯
香豆素-3-羧酸乙酯
香豆素-3-羧酸
香豆素-3-甲酰氯