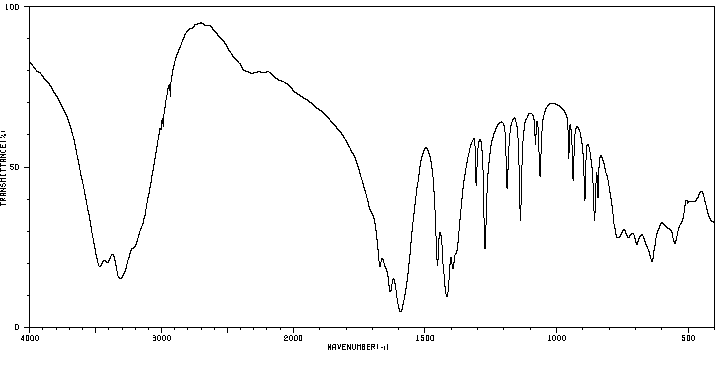
毒理性
哺乳期使用的总结:目前没有关于哺乳期间使用柠檬酸镁的临床信息。然而,已经研究了其他镁盐。静脉注射硫酸镁仅略微增加了乳汁中的镁浓度。婴儿对镁的口服吸收不良,因此母体柠檬酸镁不太可能影响哺乳婴儿的血清镁。在怀孕期间补充柠檬酸镁可能会延迟哺乳的开始,但在哺乳期间可以服用,不需要特别的预防措施。
对哺乳婴儿的影响:50位处于分娩后第一天的母亲接受了15毫升的矿物油或矿物油和另一种镁盐的乳液,即相当于900毫克氢氧化镁的氢氧化镁,尽管没有说明具体有多少人接受了每种产品。如果需要,随后的几天可以给予额外的剂量。所有哺乳的婴儿都没有出现明显异常的大便,但所有婴儿也接受了补充喂养。
对哺乳和乳汁的影响:一位因妊娠期高血压接受3天静脉注射硫酸镁的母亲,哺乳II期延迟到分娩后第10天。没有发现延迟的其他具体原因,尽管没有进行完整的评估。随后的对照临床试验发现,接受静脉注射硫酸镁治疗的母亲没有延迟哺乳的证据。一些但不是所有的研究发现,由于镁通过胎盘传递给胎儿,接受分娩期间静脉注射硫酸镁的母亲的孩子在第一次喂养的时间或吸吮能力方面有延长的趋势。
在一项对40对匹配的健康女性进行研究,这些女性经阴道分娩单胎妊娠,比较了那些在分娩前至少4周每天持续口服盐酸天冬氨酸镁补充剂(平均剂量为459毫克,每日范围365至729毫克镁)的妇女与对照组的结局终点。在镁组中,能够在出院时完全用母乳喂养婴儿的妇女明显较少(63%对80%)。
◉ Summary of Use during Lactation:No information is available on the clinical use of magnesium citrate during breastfeeding. However, other magnesium salts have been studied. Intravenous magnesium sulfate increases milk magnesium concentrations only slightly. Oral absorption of magnesium by the infant is poor, so maternal magnesium citrate is not expected to affect the breastfed infant's serum magnesium. Magnesium citrate supplementation during pregnancy might delay the onset of lactation, but it can be taken during breastfeeding and no special precautions are required.
◉ Effects in Breastfed Infants:Fifty mothers who were in the first day postpartum received 15 mL of either mineral oil or an emulsion of mineral oil and another magnesium salt, magnesium hydroxide equivalent to 900 mg of magnesium hydroxide, although the exact number who received each product was not stated. Additional doses were given on subsequent days if needed. None of the breastfed infants were noted to have any markedly abnormal stools, but all of the infants also received supplemental feedings.
◉ Effects on Lactation and Breastmilk:One mother who received intravenous magnesium sulfate for 3 days for pregnancy-induced hypertension had lactogenesis II delayed until day 10 postpartum. No other specific cause was found for the delay, although a complete work-up was not done. A subsequent controlled clinical trial found no evidence of delayed lactation in mothers who received intravenous magnesium sulfate therapy. Some, but not all, studies have found a trend toward increased time to the first feeding or decreased sucking in infants of mothers treated with intravenous magnesium sulfate during labor because of placental transfer of magnesium to the fetus.
A study in 40 pairs of matched healthy women with vaginally delivered singleton pregnancies, outcome endpoints were compared in those receiving continuous oral magnesium aspartate HCl supplementation mean dose of 459 mg daily (range 365 to 729 mg of magnesium daily) for at least 4 weeks before delivery versus non-supplemented controls. In the magnesium group, significantly fewer women could breastfeed their infants exclusively at discharge (63% vs 80%).
来源:Drugs and Lactation Database (LactMed)