2,2’-硫代二乙酰胺 | 14618-65-6
中文名称
2,2’-硫代二乙酰胺
中文别名
2,2'-硫代二乙酰胺;亚硫基二乙酰胺;2,2"-硫代二乙酰胺
英文名称
2,2'-thiodiethanamide
英文别名
2,2'-Thiobisacetamide;2-(2-amino-2-oxoethyl)sulfanylacetamide
CAS
14618-65-6
化学式
C4H8N2O2S
mdl
——
分子量
148.186
InChiKey
KQNFZEVUCSXNTH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:172-176℃
-
沸点:436.9±30.0 °C(Predicted)
-
密度:1.345±0.06 g/cm3(Predicted)
-
LogP:-1.320 (est)
-
稳定性/保质期:
在常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):-1.2
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:112
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险类别码:R20/21/22
-
海关编码:2930909090
-
安全说明:S22,S36/37
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2,2'-Sulfinylbisacetamid 97583-15-8 C4H8N2O3S 164.185
反应信息
-
作为反应物:描述:参考文献:名称:Kumar, Anil; Tilak, B. D., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1987, vol. 26, # 1-12, p. 599 - 601摘要:DOI:
-
作为产物:描述:参考文献:名称:Structure–reactivity studies on ˙OH radical reaction with substituted dialkyl sulfides摘要:本文报告的ËOH自由基与多种功能化有机硫化物的反应结果表明,pH、功能基团的性质以及链长会影响ËOH自由基与硫化物的反应特性。反应中ËH原子和ËOH自由基与2-(甲硫基)乙醇和2-(乙硫基)乙醇的水溶液反应生成的瞬态吸收光谱(λmax = 295 nm)被归为α-硫自由基。它以二级动力学衰减,且2k = 5.2 × 10^9 dm3 mol–1 s–1,且被氧气淬灭。在反应中获得的瞬态吸收光谱(λmax = 480和500 nm,pH = 1)被归为以硫为中心的二聚自由基阳离子。瞬态吸光度随pH变化在pH = 2.1处出现拐点。ËOH自由基(pH = 6)与2,2′-硫代二乙酰氯反应显示形成α-硫自由基(λmax = 300 nm)和OH-加合物(λ = 350–380 nm),而在酸性溶液中,瞬态光谱(λmax = 340 nm,τ = 0.8 µs)被归为具有4元环结构的分子内自由基阳离子。在反应中生成的瞬态物种(λmax = 370 nm,τ = 17 µs)圈定为3,3′-硫代二丙酰氯与ËOH自由基反应形成的5元环分子内自由基阳离子,并与pH无关。3,3′-硫代二丙酰胺的OH-加合物(λmax = 350 nm,pH = 11)被观察到转变为分子内自由基阳离子(λmax = 370 nm)。在酸性溶液中未观察到该转变,且在脉冲后立即观察到的只有分子内自由基阳离子(λmax = 370 nm)。2,2′-硫代二乙酰胺的α-硫自由基的贡献随pH降低,而在中性溶液中,即观察到ËOH自由基主要通过OH-加合物的形成反应,而在酸性溶液中(pH = 1)则推测出具有4元环结构的分子内自由基阳离子(λmax = 335 nm)为瞬态物种。DOI:10.1039/a900830f
文献信息
-
Die Bestimmung organischer Sulfide作者:H. Möhrle、G. HempelDOI:10.1002/ardp.19733061205日期:——Die quantitative Bestimmung organischer Sulfide gelingt mit Perjodat unter Bildung von Sulfoxiden. Bei Mercaptoverbindungen, die in α‐Stellung eine Hydroxy‐ oder Aminogruppe tragen, erfolgt keine glykolanaloge Spaltung, sondern die Oxidation zur Sulfonsäure.
-
Role of solute structure on the stability of the OH-adduct of substituted organic sulfides and its transformation to a radical cation作者:V.B. Gawandi、Hari Mohan、Jai P. MittalDOI:10.1016/s0009-2614(99)00946-x日期:1999.12to a sulfur-centered dimer radical cation (λmax=485 nm) in basic solution. The rate of transformation is accelerated in acidic solution and only the dimer radical cation is observed at pH=1. The transformation of the OH-adduct of 3-(methylthio)propanol is fast even in basic solution and only the intra-molecular radical (λmax=410 nm) is observed at pH>5 and the dimer radical cation (λmax=480 nm) at pH=1硫为中心的OH加合物(λ最大= 360纳米)的4-(甲硫基)丁醇变换(ķ = 1×10 6小号-1),以硫为中心的二聚体自由基阳离子(λ最大在基本= 485 nm)的解决方案。在酸性溶液中,转化速率加快,在pH = 1时仅观察到二聚自由基阳离子。3-(甲硫基)丙醇的OH加合物的变换是快,即使在碱性溶液和仅分子内自由基(λ最大= 410纳米)在pH> 5和二聚体自由基阳离子观察到(λ最大= 480 pH = 1时)。3,3'- thiodipropionamide(的OH加合物λ最大= 350纳米)经历变换(ķ = 1.4×10 5小号-1),以分子内自由基阳离子(λ最大= 370纳米)。用2-(甲硫基)乙醇和2,2'-硫代二乙酰胺无法观察到OH-加合物的形成及其向溶质自由基阳离子的转化。
-
Solladie-Cavallo,A.; Vieles,P., Bulletin de la Societe Chimique de France, 1967, p. 517 - 523作者:Solladie-Cavallo,A.、Vieles,P.DOI:——日期:——
-
Schulze,E., Zeitschrift fur Chemie, 1865, p. 73作者:Schulze,E.DOI:——日期:——
-
Kumar, Anil; Tilak, B. D., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1986, vol. 25, p. 880 - 882作者:Kumar, Anil、Tilak, B. D.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
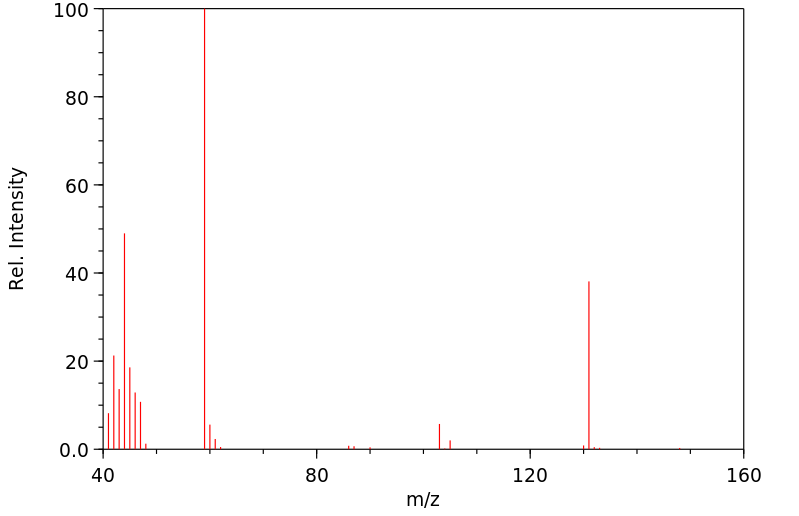
质谱MS
-
碳谱13CNMR
-
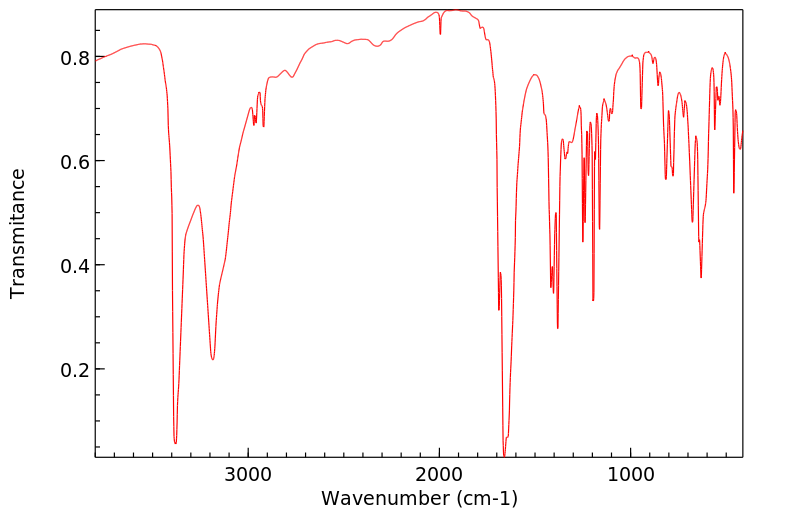
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸