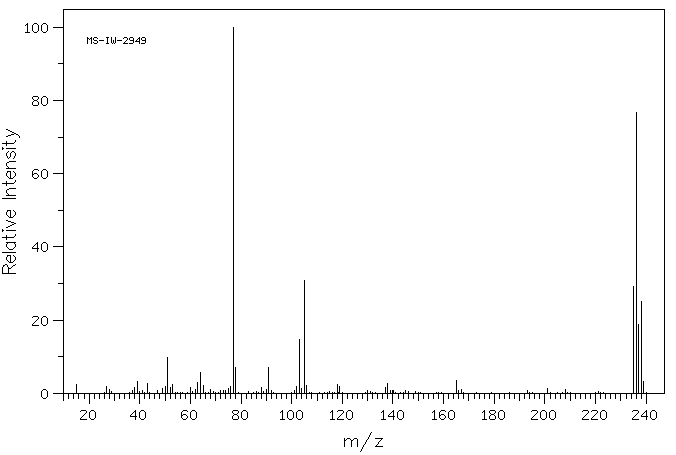
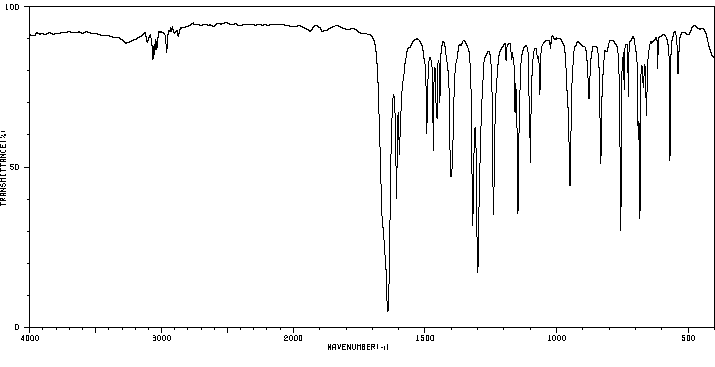
4-氯-5-甲氧基-2-苯基哒嗪-3-酮 | 2514-18-3
中文名称
4-氯-5-甲氧基-2-苯基哒嗪-3-酮
中文别名
——
英文名称
4-chloro-5-methoxy-2-phenyl-2H-pyridazin-3-one
英文别名
4-chloro-5-methoxy-2-phenylpyridazin-3(2H)-one;4-chloro-5-methoxy-2-phenyl-3(2H)-pyridazinone;1-Phenyl-4-methoxy-5-chlor-6(1H)-pyridazinon;1-Fenyl-4-methoxy-5-chlorpyridazon-6;4-Chlor-5-methoxy-2-phenyl-3-oxo-2,3-dihydro-pyridazin;2-phenyl-4-chloro-5-methoxy-3(2H)-pyridazinone;2-phenyl-4-chloro-5-methoxypyridazin-3-one;SKI-104386;4-chloro-5-methoxy-2-phenylpyridazin-3-one
CAS
2514-18-3
化学式
C11H9ClN2O2
mdl
MFCD00096938
分子量
236.658
InChiKey
RSTBYGMJEKPLBW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160-161 °C
-
沸点:366.8±52.0 °C(Predicted)
-
密度:1.31±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:41.9
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-苯基-4,5-二氯-6-哒酮 4,5-dichloro-1-phenylpyridazin-6-one 1698-53-9 C10H6Cl2N2O 241.076 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 D咪唑并n 1-Phenyl-4,5-dimethoxy-6-pyridazon 3295-78-1 C12H12N2O3 232.239
反应信息
-
作为反应物:描述:参考文献:名称:2,5-dihydro-5-aryliminopyrrole derivatives and their preparation摘要:2,5-二氢-5-芳基亚胺吡咯衍生物的化学式为##STR1##其中R.sup.1为氢或烷基,R.sup.2和R.sup.3分别为烷基,Ar为未取代或取代的芳基,用于控制不良植物生长。公开号:US04585881A1
-
作为产物:描述:参考文献:名称:Prostaglandin endoperoxide H synthase biosynthesis inhibitors摘要:该发明描述了式I的吡啶并酮化合物,这些化合物是环氧合酶(COX)抑制剂,特别是选择性地抑制环氧合酶-2(COX-2)。COX-2是与炎症相关的可诱导异构体,与构成性异构体环氧合酶-1(COX-1)相对,后者是许多组织中重要的“基础”酶,包括胃肠道(GI)和肾脏。这些化合物对COX-2的选择性减少了目前市售的非甾体类抗炎药(NSAIDs)所见到的不良胃肠道和肾脏副作用。公开号:US06307047B1
-
作为试剂:描述:4,5-dichloro-1-(phenyl)-3(2H)-pyridazinone 、 1-苯基-4,5-二氯-6-哒酮 、 potassium carbonate 在 4-氯-5-甲氧基-2-苯基哒嗪-3-酮 、 水 、 乙酸乙酯 、 乙酸盐 、 Brine 作用下, 以 甲醇 为溶剂, 反应 5.0h, 以to provide 2-phenyl-4-chloro-5-methoxy-3(2H)-pyridazinone (yield: 920 mg, 95%)的产率得到4-氯-5-甲氧基-2-苯基哒嗪-3-酮参考文献:名称:Prostaglandin endoperoxide H synthase biosynthesis inhibitors摘要:本发明描述了公式I的吡啶并咪唑酮化合物,它们是环氧合酶(COX)抑制剂,特别是选择性抑制剂环氧合酶-2(COX-2),COX-2是与炎症相关的诱导型同工酶,与组成型同工酶环氧合酶-1(COX-1)相反,后者是许多组织中重要的“管家”酶,包括胃肠道和肾脏。这些化合物对COX-2的选择性最小化了当前标记的非甾体抗炎药(NSAIDs)所见的不良胃肠和肾脏副作用。公开号:US07115591B2
文献信息
-
[EN] PROSTAGLANDIN ENDOPEROXIDE H SYNTHASE BIOSYNTHESIS INHIBITORS<br/>[FR] INHIBITEURS DE LA BIOSYNTHESE DE LA PROSTAGLANDINE ENDOPEROXYDE H SYNTHASE申请人:ABBOTT LAB公开号:WO2000024719A1公开(公告)日:2000-05-04The present invention describes pyridazinone compounds of formula (I) which are cyclooxygenase (COX) inhibitors, and in particular, are selective inhibitors of cyclooxygenase-2 (COX-2). COX-2 is the inducible isoform associated with inflammation, as opposed to the constitutive isoform, cyclooxygenase-1 (COX-1) which is an important 'housekeeping' enzyme in many tissues, including the gastrointestinal (GI) tract and the kidneys. The selectively of these compounds for COX-2 minimizes the unwanted GI and renal side-effects seen with currently marketed non-steroidal anti-inflammatory drugs (NSAIDs).
-
PYRIDAZINONES AND FURAN-CONTAINING COMPOUNDS申请人:Djaballah Hakim公开号:US20100210649A1公开(公告)日:2010-08-19The present invention is directed to pyridazinone compounds of formula (I) and furan compounds of formula (II), pharmaceutical compositions of compounds of formula (I) and (II), kits containing these compounds, methods of syntheses, and a method of treatment of a proliferative disease in a subject by administration of a therapeutically effective amount of a compound of formulae (I) or (II). Both classes of compounds were identified through screening of a collection of small molecule libraries.
-
Synthesis and Biological Evaluation of Novel Pyridazinone-Based α<sub>4</sub> Integrin Receptor Antagonists作者:Yong Gong、J. Kent Barbay、Alexey B. Dyatkin、Tamara A. Miskowski、Edward S. Kimball、Stephen M. Prouty、M. Carolyn Fisher、Rosemary J. Santulli、Craig R. Schneider、Nathaniel H. Wallace、Scott A. Ballentine、William E. Hageman、John A. Masucci、Bruce E. Maryanoff、Bruce P. Damiano、Patricia Andrade-Gordon、Dennis J. Hlasta、Pamela J. Hornby、Wei HeDOI:10.1021/jm060031q日期:2006.6.1A novel series of pyridazinone-functionalized phenylalanine analogues was prepared and evaluated for inhibition of cellular adhesion mediated by alpha(4)beta(1)/VCAM-1 and alpha(4)beta(7)/MAdCAM-1 interactions. Concise syntheses were developed and applied for exploration of structure-activity relationships pertaining to the pyridazinone ring as well as the N-acyl phenylalanine scaffold. Potent dual antagonists of alpha(4)beta(1) and alpha(4)beta(7) were generated from an amide subseries; antagonists selective for alpha(4)beta(7) were identified from urea and carbamate-based subseries. The pharmacokinetic properties of selected members of the series have been determined in rats and demonstrate that the use of ester prodrugs and alterations to the amide linkage can lead to improved oral bioavailability in this series. An alpha(4),beta(7)-selective member of the carbamate subseries (36c), upon oral admininstration, demonstrated in vivo efficacy in the mouse DSS colitis model.
-
Lyga, John W., Journal of Heterocyclic Chemistry, 1988, vol. 25, p. 1757 - 1760作者:Lyga, John W.DOI:——日期:——
-
Suzuki reactions on chloropyridazinones: an easy approach towards arylated 3(2 H )-pyridazinones作者:Bert U.W Maes、Omar R'kyek、Janez Košmrlj、Guy L.F Lemière、Eddy Esmans、Jef Rozenski、Roger A Dommisse、Achiel HaemersDOI:10.1016/s0040-4020(00)01100-5日期:2001.2The synthesis of 4-aryl-5-methoxy-, 5-aryl-4-methoxy- and 4,5-diaryl-3(2H)-pyridazinones via Suzuki palladium-catalysed cross-coupling reactions with the corresponding chloro-3(2H)-pyridazinones is described. (C) 2001 Elsevier Science Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3