9-甲基-3-硝基-9h-咔唑 | 61166-05-0
中文名称
9-甲基-3-硝基-9h-咔唑
中文别名
——
英文名称
9-methyl-3-nitro-9H-carbazole
英文别名
3-nitro-N-methyl carbazole;3-nitro-9-methylcarbazole;9-methyl-3-nitrocarbazole;9-methyl-3-nitro-carbazole;9-Methyl-3-nitro-carbazol;3-Nitro-9-methyl-carbazol
CAS
61166-05-0
化学式
C13H10N2O2
mdl
MFCD00218829
分子量
226.235
InChiKey
LUWUTJWWCMXMSA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:172 °C
-
溶解度:<0.2 [ug/mL]
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:17
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:50.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2933990090
SDS
| Name: | 9-Methyl-3-nitro-9h-carbazole 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 61166-05-0 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 61166-05-0 | 9-Methyl-3-nitro-9H-carbazole | 97% | unlisted |
Risk Phrases: 68
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Possible risk of irreversible effects.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Possible risk of irreversible effects.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 61166-05-0: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 172 - 173.5 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C13H10N2O2
Molecular Weight: 226
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 61166-05-0: FE6270000 LD50/LC50:
Not available.
Carcinogenicity:
9-Methyl-3-nitro-9H-carbazole - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 68 Possible risk of irreversible effects.
Safety Phrases:
S 36/37 Wear suitable protective clothing and
gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 61166-05-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 61166-05-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 61166-05-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-硝基-9h-咔唑 3-Nitro-carbazol 3077-85-8 C12H8N2O2 212.208 3-硝基-9-硝基咔唑 3-nitro-9-nitroso-carbazole 5393-41-9 C12H7N3O3 241.206 N-甲基咔唑 N-methylcarbazole 1484-12-4 C13H11N 181.237 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 9-甲基咔唑-3-胺 3-amino-N-methylcarbazole 61166-04-9 C13H12N2 196.252 —— 3-(N-methylamino)-9-methylcarbazole 5416-98-8 C14H14N2 210.279 —— Bis-(9-methyl-9H-carbazol-3-yl)-diazene 95600-13-8 C26H20N4 388.472 —— 3-(N,N-dimethylamino)-9-methylcarbazole 94127-15-8 C15H16N2 224.305 —— N-(9-methyl-carbazol-3-yl)-acetamide 180331-84-4 C15H14N2O 238.289 —— 9-methyl-3,4-diaminocarbazole 138963-62-9 C13H13N3 211.266 —— trifluoro-acetic acid-(9-methyl-carbazol-3-ylamide) 318-11-6 C15H11F3N2O 292.26 —— N-(9-methylcarbazol-3-yl)-2,2-dimethylpropionamide 373367-61-4 C18H20N2O 280.37 —— 3-(N,N-dimethylamino)-4-nitro-9-methylcarbazole 94127-25-0 C15H15N3O2 269.303
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis and Ultraviolet Absorption Spectra Studies of 2,3- and 3,4-Disubstituted Carbazoles1摘要:DOI:10.1021/ja01632a009
-
作为产物:描述:参考文献:名称:Stevens; Tucker, Journal of the Chemical Society, 1923, vol. 123, p. 2147摘要:DOI:
文献信息
-
Iodine(III)-Catalyzed Electrophilic Nitration of Phenols via Non-Brønsted Acidic NO<sub>2</sub><sup>+</sup> Generation作者:Kevin A. Juárez-Ornelas、J. Oscar C. Jiménez-Halla、Terumasa Kato、César R. Solorio-Alvarado、Keiji MaruokaDOI:10.1021/acs.orglett.8b04141日期:2019.3.1The first catalytic procedure for the electrophilic nitration of phenols was developed using iodosylbenzene as an organocatalyst based on iodine(III) and aluminum nitrate as a nitro group source. This atom-economic protocol occurs under mild, non-Brønsted acidic and open-flask reaction conditions with a broad functional-group tolerance including several heterocycles. Density functional theory (DFT)
-
3-Aminocarbazole Compounds, Pharmaceutical Composition Containing the Same and Method for the Preparation Thereof申请人:Alisi Maria Alessandra公开号:US20080207727A1公开(公告)日:2008-08-28A compound of formula (I), in which R1, R2, R3, R4, R5, R6, X and Y have the meanings indicated in the description, and the pharmaceutically acceptable salts thereof. A pharmaceutical composition containing a compound of formula (I) or a pharmaceutically acceptable salt thereof. A method for preparing the abovementioned compound of formula (I) and the pharmaceutically acceptable salts thereof.公式(I)的化合物,其中R1、R2、R3、R4、R5、R6、X和Y具有描述中指示的含义,以及其药学上可接受的盐。含有公式(I)的化合物或其药学上可接受的盐的药物组合物。制备上述公式(I)化合物及其药学上可接受的盐的方法。
-
Synthesis of Novel 3-<i>N</i> -substituted Carbazole Derivatives and Evaluation of their Abilities to Inhibit Platelet Aggregation作者:Jiseon Kim、Sang-Hyuk Jung、Eunju Yun、Soo-Hyun Cho、O Yuseok、Ji-Eun Kim、Young-Ho Kim、Chang-Seon Myung、Gyu-Yong SongDOI:10.1002/bkcs.11462日期:2018.6activities, including anticancer, antiviral, anti‐inflammatory, and antimicrobial activities; some compounds containing the moiety also inhibit platelet aggregation. In the present study, we synthesized a series of 3‐N‐substituted carbazole derivatives and evaluated their abilities to inhibit in vitro platelet aggregation induced by collagen (5 μg/mL). Of the synthesized compounds, compound 5q (JSCa15), with咔唑部分具有多种生物学活性,包括抗癌,抗病毒,抗炎和抗微生物活性。一些含有该部分的化合物也抑制血小板凝集。在本研究中,我们合成了一系列3 N取代的咔唑衍生物,并评估了它们抑制胶原蛋白(5μg/ mL)诱导的体外血小板聚集的能力。在合成的化合物中,在咔唑骨架内具有脲键的化合物5q(JSCa15)表现出最强的抑制活性(30μM时为98.25%)。有趣的是,化合物5q的硝基还原为胺后,其活性大大降低(化合物5r,在30μM时为5.18%)。同样,用氨基甲酸酯部分代替化合物5q的脲部分导致活性的显着损失(化合物8a,在30μM时5.91%)。这些结果表明,3 N取代的咔唑衍生物的尿素部分和硝基可能在抑制胶原蛋白诱导的体外血小板凝集中起关键作用。
-
Regioselective Functionalization of 9,9-Dimethyl-9-silafluorenes by Borylation, Bromination, and Nitration作者:Masahito Murai、Naoki Nishinaka、Mizuki Kimura、Kazuhiko TakaiDOI:10.1021/acs.joc.9b00598日期:2019.5.3Despite the utility of 9-silafluorenes as functional materials and as building blocks, methods for efficient functionalization of their backbone are rare, probably because of the presence of easily cleavable C–Si bonds. Although controlling the regioselectivity of iridium-catalyzed direct borylation of C–H bonds is difficult, we found that bromination and nitration of 2-methoxy-9-silafluorene under
-
METHOD FOR PRODUCING AMINOCARBAZOLES申请人:——公开号:US20010049448A1公开(公告)日:2001-12-06Disclosed is a method for producing an aminocarbazole by catalytically reducing a nitrocarbazole, wherein as a catalyst is used an active nickel-based catalyst prepared by contacting a nickel-based catalyst with an alkali and an iron compound under a hydrogen gas or inert gas atmosphere in an inert solvent, and according to the method of the present inventions decrease in reaction speed, decrease in yield of a product or the like depending upon the lot of the starting nitrocarbazoles can be prevented, and aminocarbazoles can be produced in a good yield constantly.
表征谱图
-
氢谱1HNMR
-
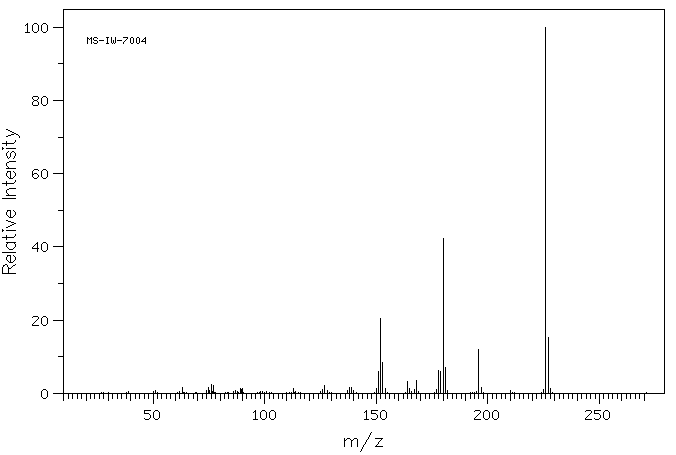
质谱MS
-
碳谱13CNMR
-
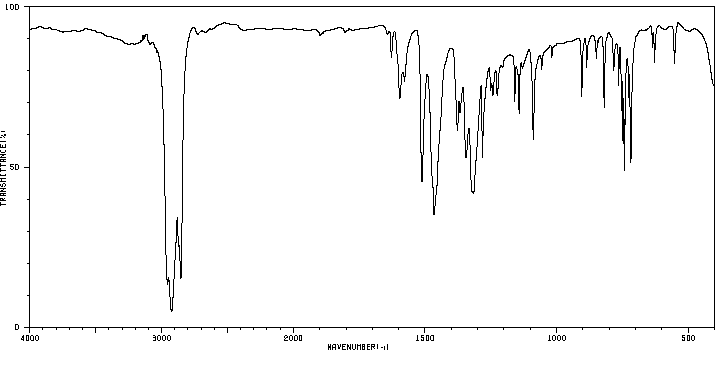
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3