6-碘烟酸甲酯 | 173157-33-0
中文名称
6-碘烟酸甲酯
中文别名
——
英文名称
methyl 6-iodonicotinate
英文别名
methyl 6-iodo-3-pyridinecarboxylate;methyl 6-iodopyridine-3-carboxylate
CAS
173157-33-0
化学式
C7H6INO2
mdl
——
分子量
263.035
InChiKey
CGFVEUUNDFAFHE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:287.7±25.0 °C(Predicted)
-
密度:1.844±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:39.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2933399090
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Methyl 6-iodonicotinate
Synonyms: Methyl 2-iodopyridine-5-carboxylate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 6-iodonicotinate
CAS number: 173157-33-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H6INO2
Molecular weight: 263.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen Iodide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Methyl 6-iodonicotinate
Synonyms: Methyl 2-iodopyridine-5-carboxylate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 6-iodonicotinate
CAS number: 173157-33-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H6INO2
Molecular weight: 263.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen Iodide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:Bi-aromatic compounds linked via a heteroethylene radical, and pharmaceutical and cosmetic compositions using them摘要:提供了通过异乙炔基团连接的双芳香化合物,以及含有这些化合物的药用和化妆品组合物,以及它们的使用方法。公开号:US06818666B2
-
作为产物:描述:methyl 2-<(trifluoromethyl)sulfonyl>pyridine-5-carboxylate 在 盐酸 、 sodium iodide 、 sodium hydroxide 作用下, 以 乙醚 、 甲苯 、 水 为溶剂, 以1.67 g的产率得到6-碘烟酸甲酯参考文献:名称:羟基吡啶的一锅法碘化摘要:描述了羟基吡啶和羟基喹啉的一锅高产率的碘化。碘化反应在温和的条件下进行,不需要色谱纯化就可以高收率获得产物。另外,碘化作用在2-和4-羟基吡啶和-羟基喹啉上。DOI:10.1021/jo900726f
文献信息
-
[EN] SUBSTITUTED 3-AMINO-THIENO[2,3-B] PYRIDINE-2-CARBOXYLIC ACID AMIDE COMPOUNDS AS IKK INHIBITORS<br/>[FR] COMPOSES AMIDE D'ACIDE 3-AMINO-THIENO[2,3-B] PYRIDINE-2-CARBOXYLIQUE SUBSTITUE SERVANT D'INHIBITEURS D'IKK申请人:BOEHRINGER INGELHEIM PHARMA公开号:WO2005056562A1公开(公告)日:2005-06-23Disclosed are compounds of formula (I): wherein the variables R1, R2, R3 and Z are described herein, which are useful as inhibitors of the kinase activity of the IκB kinase (IKK) complex. The compounds are therefore useful in the treatment of IKK mediated diseases including autoimmune diseases inflammatory diseases and cancer. Also disclosed are pharmaceutical compositions comprising these compounds and processes for preparing these compounds.披露了公式(I)的化合物:其中变量R1、R2、R3和Z如本文所述,它们作为IκB激酶(IKK)复合物的激酶活性的抑制剂是有用的。因此,这些化合物在治疗IKK介导的疾病,包括自身免疫性疾病、炎症性疾病和癌症方面是有用的。还披露了包含这些化合物的药物组合物以及制备这些化合物的方法。
-
TCDA: Practical Synthesis and Application in the Trifluoromethylation of Arenes and Heteroarenes作者:Jian Wang、Xiaomin Zhang、Zehong Wan、Feng RenDOI:10.1021/acs.oprd.6b00079日期:2016.4.15A practical synthesis of the reagent trimethylsilyl chlorodifluoroacetate (TCDA) is reported on 50 g scale. The trifluoromethylation with TCDA was optimized, and the reaction shows very broad scope with respect to electron-deficient, -neutral, -rich aryl/heteroaryl iodides, as well as excellent functional group tolerability, such as ester, amide, aldehyde, hydroxyl, and carboxylic acid. The reagent
-
[EN] N-ALKYL PYRROLES AS HMG-COA REDUCTASE INHIBITORS<br/>[FR] PYRROLES DE N-ALKYL COMME INHIBITEURS DE L'HMG-COA-REDUCTASE申请人:WARNER LAMBERT CO公开号:WO2005056004A1公开(公告)日:2005-06-23HMGCo-A reductase inhibitor compounds useful as hypocholesterolemic and hypolipidemic compounds are provided. Also provided are pharmaceutical compositions of the compounds. Methods of making and methods of using the compounds are also provided. Formula (I).
-
Palladium-catalyzed difluoromethylthiolation of heteroaryl bromides, iodides, triflates and aryl iodides作者:Jiang Wu、Yafei Liu、Changhui Lu、Qilong ShenDOI:10.1039/c6sc00082g日期:——A palladium-catalyzed difluoromethylthiolation of heteroaryl halides and triflates under mild conditions was described. A vast range of heteroaryl halides such as pyridyl, quinolinyl, benzothiazolyl, thiophenyl, carbazolyl and pyazolyl halides could be difluoromethylthiolated efficiently, thus providing medicinal chemists with new tools for their search of new lead compounds for drug discovery. Likewise
-
Adamantyl-substituted polycyclic acetylene compounds and申请人:Centre International de Recherches Dermatologiques Galderma公开号:US05574036A1公开(公告)日:1996-11-12Novel pharmaceutically/cosmetically-active adamantyl-substituted polycyclic acetylene compounds have the structural formula (I): ##STR1## wherein Ar is a radical having one of the formulae (a)-(f): ##STR2## and are useful for the treatment of a wide variety of disease states, whether human or veterinary, for example dermatological, rheumatic, respiratory, cardiovascular, bone and ophthalmological disorders, as well as for the treatment of mammalian skin and hair conditions/disorders.新型具有药用/化妆活性的脂环己基取代多环乙炔化合物具有结构式(I):其中Ar是具有式(a)-(f)之一的基团,对于治疗广泛的疾病状态是有用的,无论是人类还是兽医,例如皮肤病、风湿病、呼吸系统疾病、心血管疾病、骨骼疾病和眼科疾病,以及治疗哺乳动物的皮肤和毛发状况/疾病。
表征谱图
-
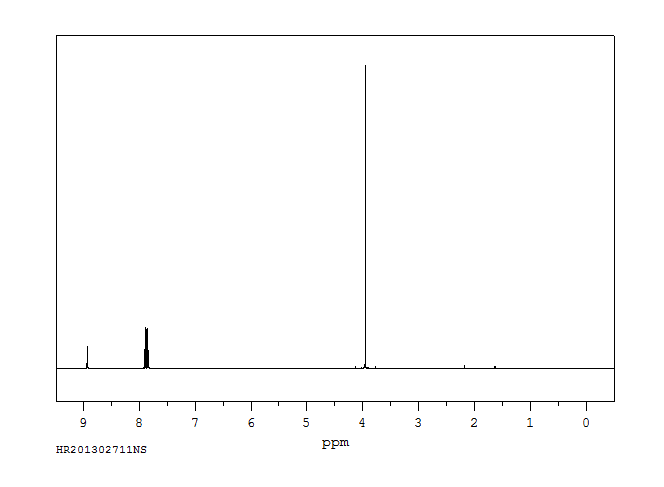
氢谱1HNMR
-
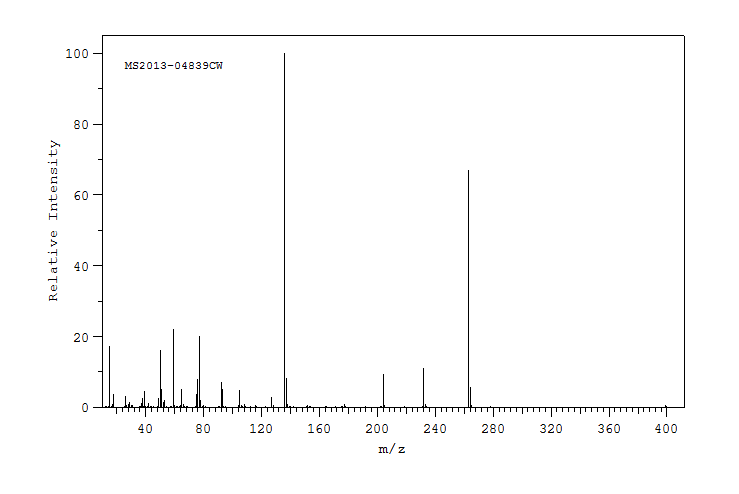
质谱MS
-
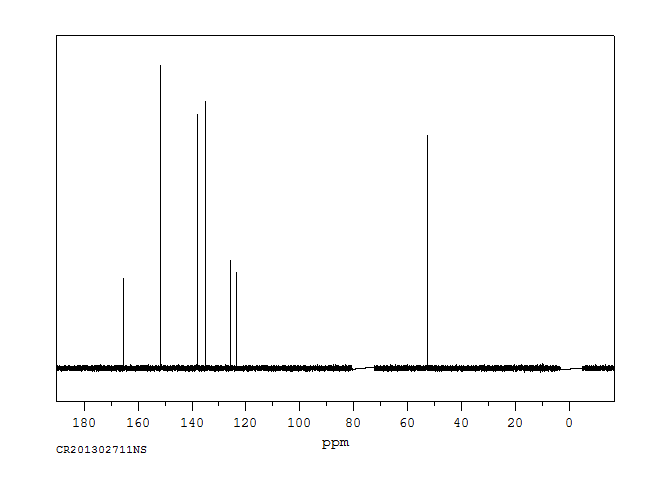
碳谱13CNMR
-
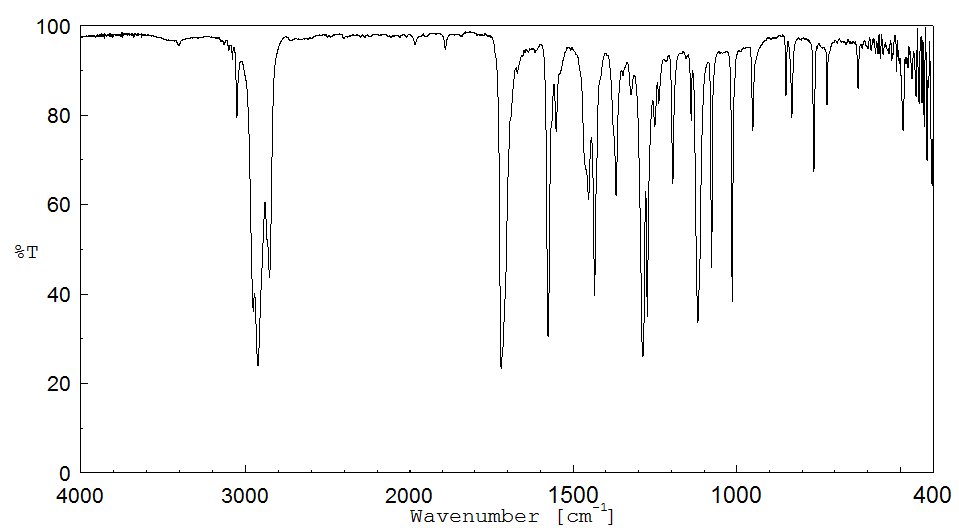
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-