p-二甲氨基苄烯-p-对氨基苯乙醚 | 15484-93-2
中文名称
p-二甲氨基苄烯-p-对氨基苯乙醚
中文别名
——
英文名称
N-<(4-dimethylamino)benzylidene>-4-ethoxyaniline
英文别名
N-<4-(dimethylamino)benzylidene>-4-ethoxyaniline;N--p-phenetidine;N-(4-dimethylamino-benzylidene)-p-phenetidine;N-(4-Dimethylamino-benzyliden)-p-phenetidin;4-Dimethylamino-benzaldehyd-(4-aethoxy-anil);(4-Dimethylamino-benzal)-p-phenetidin;p-Dimethylaminobenzylidene p-phenetidine;4-[(4-ethoxyphenyl)iminomethyl]-N,N-dimethylaniline
CAS
15484-93-2
化学式
C17H20N2O
mdl
——
分子量
268.359
InChiKey
ULZYQFCXFXYXBU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:151 °C
-
沸点:424.1±30.0 °C(Predicted)
-
密度:0.99±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:20
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.235
-
拓扑面积:24.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2925290090
SDS
反应信息
-
作为反应物:描述:p-二甲氨基苄烯-p-对氨基苯乙醚 在 盐酸 、 溶剂黄146 作用下, 以 甲醇 、 乙醇 为溶剂, 反应 12.0h, 生成 (4-Dimethylamino-phenyl)-(4-ethoxy-phenylamino)-acetic acid; hydrochloride参考文献:名称:Naim, S. Shawkat; Khan, Naseem H.; Siddiqui, Amin A., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1980, vol. 19, # 7, p. 622 - 624摘要:DOI:
-
作为产物:描述:参考文献:名称:Sachs; Lewin, Chemische Berichte, 1902, vol. 35, p. 3577摘要:DOI:
文献信息
-
Three-component synthesis of chromeno β-lactam hybrids for inflammation and cancer screening作者:Nassim Borazjani、Saghi Sepehri、Maryam Behzadi、Aliasghar Jarrahpour、Javad Ameri Rad、Maryam Sasanipour、Milad Mohkam、Younes Ghasemi、Amin Reza Akbarizadeh、Carole Digiorgio、Jean Michel Brunel、Mohammad Mehdi Ghanbari、Gyula Batta、Edward TurosDOI:10.1016/j.ejmech.2019.06.036日期:2019.10Highly diastereoselective synthesis of chromeno β-lactam hybrids was achieved by an efficient one-pot three-component reaction. With this procedure, the desired β-lactam products were obtained in good yields and with exclusive cis stereoselection, by combining a variety of benzaldehydes, malononitrile, and either 5,5-dimethylcyclohexane-1,3-dione or 4-hydroxycoumarin in the presence of 1,4-diazabicyclo通过高效的一锅三组分反应,实现了铬诺β-内酰胺杂合体的高度非对映选择性合成。通过这种方法,在存在下,通过将各种苯甲醛,丙二腈和5,5-二甲基环己烷-1,3-二酮或4-羟基香豆素结合使用,可以以高收率和独有的顺式立体选择获得所需的β-内酰胺产物在回流条件下得到1,4-二氮杂双环[2.2.2]辛烷。这些加合物的结构基于IR,1D和2D NMR光谱,X射线分析,H–H COZY和H–C HSQC二维NMR实验以及元素分析。筛选每种合成的化合物的抗炎和抗癌活性。β-内酰胺5b和8b分别显示出53.4和19.8的抗炎率,而5b则比用于治疗类风湿和皮肤炎症的众所周知的地塞米松皮质类固醇更有活性。β-内酰胺5a,5b,5e,5f,5g,8c,8j和8p也显示出对SW1116(结肠癌)细胞系的良好抗肿瘤活性,而对HepG2对照细胞系没有明显的细胞毒性。
-
Araya, K., Molecular Crystals and Liquid Crystals Science and Technology, Section A: Molecular Crystals and Liquid Crystals, 1994, vol. 241, p. 249 - 254作者:Araya, K.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
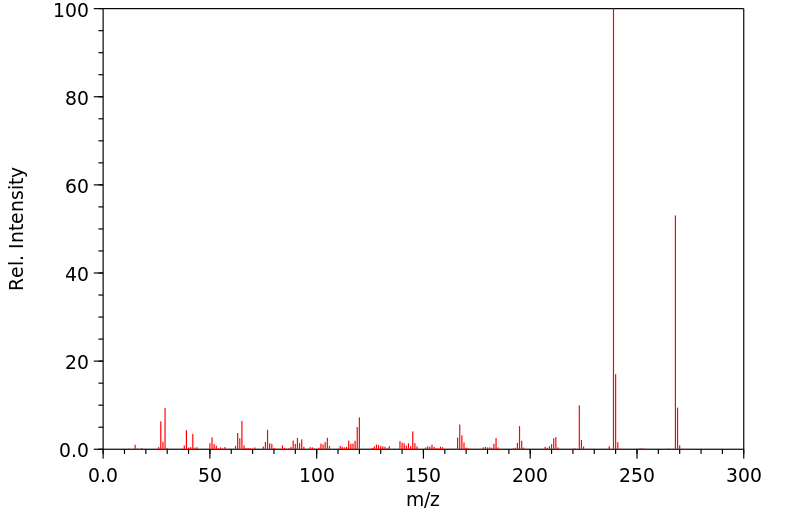
质谱MS
-
碳谱13CNMR
-
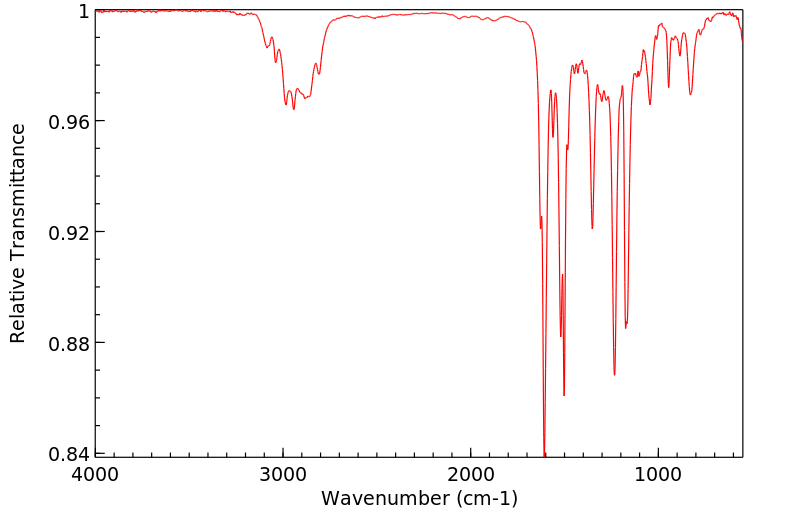
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯