2-methyl-N-(2-nitrophenyl)propanamide | 6316-52-5
中文名称
——
中文别名
——
英文名称
2-methyl-N-(2-nitrophenyl)propanamide
英文别名
N-(3-nitropyridin-2-yl)isobutyramide;2-methyl-2'-nitropropananilide;N-(2-nitrophenyl)isobutyramide;2'-Nitro-isobutyranilid
CAS
6316-52-5
化学式
C10H12N2O3
mdl
MFCD01214271
分子量
208.217
InChiKey
OBFKXTLYUKUWKH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:70-71 °C
-
沸点:392.1±25.0 °C(Predicted)
-
密度:1.241±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:15
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:74.9
-
氢给体数:1
-
氢受体数:3
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-(2-aminophenyl)isobutyramide 255735-87-6 C10H14N2O 178.234
反应信息
-
作为反应物:描述:2-methyl-N-(2-nitrophenyl)propanamide 在 5%-palladium/activated carbon 、 氢气 作用下, 以 乙醇 为溶剂, 反应 6.0h, 以96%的产率得到N-(2-aminophenyl)isobutyramide参考文献:名称:Oligoamide Foldamers家族中的折叠模式摘要:合成了一系列小的,不对称的吡啶-2,6-二羧基酰胺低聚酰胺折叠剂,它们在末端基团上具有不同的长度和取代基,以研究其构象性质和折叠模式。@型折叠模式类似于酶的氧阴离子孔基序,但也可以表征几种其他的折叠模式。计算研究表明,稳定性几乎相等的几种替代构象异构体。这些折叠模式在分子内氢键模式和芳基-芳基相互作用方面互不相同。在固态状态下,折叠器采用球状@型折叠或扩展的S型构象,这与通过计算获得的折叠器非常相似。在某些情况下,相同的折叠分子甚至可以结晶成两种不同的折叠模式,从而证实尽管形状完全不同,但不同的折叠模式在能量上却非常接近。最后,在液态下观察到的NOE相互作用的最佳匹配是一个构象,该构象与计算得出的螺旋型折叠相匹配。DOI:10.1002/chem.201406521
-
作为产物:描述:2-甲基-N-苯基丙酰胺 在 bismuth (III) nitrate pentahydrate 、 乙酸酐 作用下, 以 二氯甲烷 为溶剂, 以84%的产率得到2-methyl-N-(2-nitrophenyl)propanamide参考文献:名称:使用硝酸铋/乙酸酐对N-苯基羧酰胺和伯苯胺进行区域选择性邻位硝化摘要:用于区域选择性的有效和一釜合成方法的邻位的的-nitration Ñ苯基羧酰胺和初级苯胺已经通过使用硝酸铋和乙酸酐作为硝化试剂显影。反应在室温下进行,并以中等至极好的收率得到相应的正交硝化产物。该方法在温和条件下提供了操作简单,区域选择性和有效的途径来合成邻硝基苯胺。DOI:10.1016/j.tet.2013.08.076
文献信息
-
A Convenient Synthesis of Carboxanilides from Silyl Carboxylates and Weakly Nucleophilic Anilines Using<i>p</i>-Trifluoromethylbenzoic Anhydride and a Catalytic Amount of Active Titanium(IV) Salt作者:Mitsutomo Miyashita、Isamu Shiina、Teruaki MukaiyamaDOI:10.1246/cl.1993.1053日期:1993.6Various carboxanilides are prepared in excellent yields from nearly equimolar amounts of silyl carboxylates and the corresponding weakly nucleophilic anilines under mild conditions by using p-trifluoromethylbenzoic anhydride and a catalytic amount of active titanium(IV) salt.
-
An Effective Method for Acylation of Weakly Nucleophilic Anilines with Silyl Carboxylates via Mixed Anhydrides作者:Mitsutomo Miyashita、Isamu Shiina、Teruaki MukaiyamaDOI:10.1246/bcsj.67.210日期:1994.1In the presence of a catalytic amount of active titanium(IV) salt generated in situ from 1 mol of TiCl4 and 2 mol of AgOTf, weakly nucleophilic anilines react under mild conditions with nearly equimolar amounts of silyl carboxylates to afford the corresponding anilides in excellent yields using 4-(trifluoromethyl)benzoic anhydride. The mixed anhydride formed in situ from trimethylsily acetate and 4-(trifluoxomethyl)benzoic anhydride, a key intermediate of this reaction, was detected by 1H NMR experiment. Further, it was shown that the reaction of the mixed anhydrides and 2-nitroaniline was faster than that of the corresponding homo anhydrides and 2-nitroaniline.
-
Palladium-Catalyzed Synthesis of Aryl Amides through Silanoate-Mediated Hydrolysis of Nitriles作者:Craig Jamieson、Christopher McPherson、Keith Livingstone、Iain SimpsonDOI:10.1055/s-0035-1560724日期:——A procedure for the formation of aryl amides through the palladium-catalyzed coupling of nitriles and aryl bromides, via the formation of intermediary silanoate derived imidate species is reported. Optimization was undertaken and examples of the process are described that furnish the products in up to 86% isolated yield.
-
Synthesis of Benzimidazoles by Phosphine-Mediated Reductive Cyclisation of ortho-Nitro-anilides作者:Jan Duchek、Andrea VasellaDOI:10.1002/hlca.201100123日期:2011.6the 2‐substituted benzimidazoles 6–8 and to the imidazo[4,5‐b]pyridine 10, respectively, in yields between 45 and 85%. Heating 1 with (EtO)3P effected cyclisation and N‐ethylation, leading to the 1‐ethylbenzimidazole 6b. The slow cyclisation of the N‐pivaloylnitroaniline 2b allowed isolation of the intermediate phosphine imide 11 that slowly transformed into the 1H‐benzimidazole 7b. The structure of
-
Suzuki, Hitomi; Tatsumi, Atsuo; Ishibashi, Taro, Journal of the Chemical Society. Perkin transactions I, 1995, # 4, p. 339 - 344作者:Suzuki, Hitomi、Tatsumi, Atsuo、Ishibashi, Taro、Mori, TadashiDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
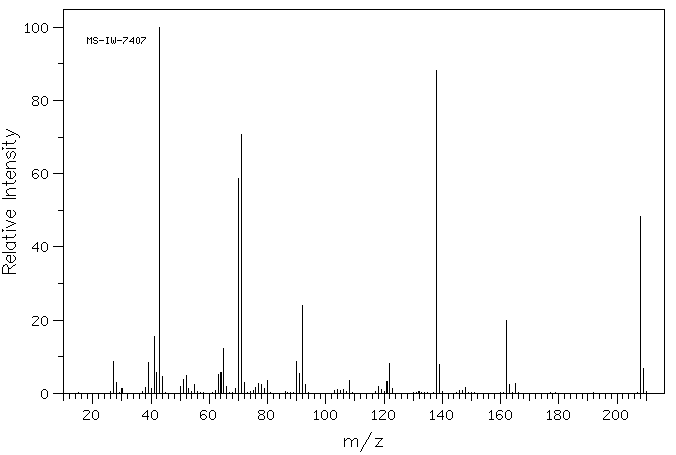
质谱MS
-
碳谱13CNMR
-
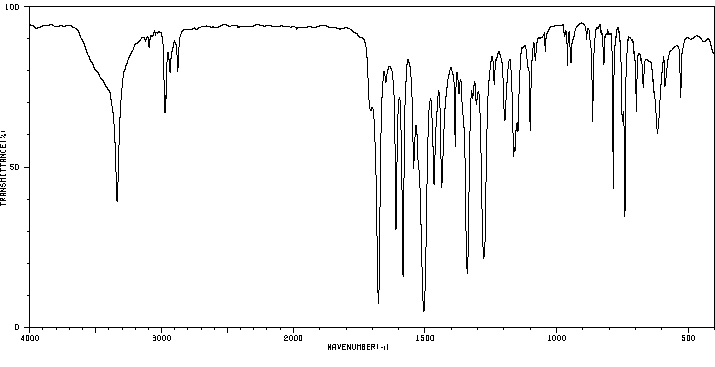
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫