DL-去甲变肾上腺素盐酸盐 | 1011-74-1
中文名称
DL-去甲变肾上腺素盐酸盐
中文别名
——
英文名称
(+/-)-Normetanephrine hydrochloride
英文别名
4-(2-Amino-1-hydroxyethyl)-2-methoxyphenol;hydron;chloride;4-(2-amino-1-hydroxyethyl)-2-methoxyphenol;hydron;chloride
CAS
1011-74-1
化学式
C9H13NO3*ClH
mdl
MFCD00012882
分子量
219.668
InChiKey
VKFPRGQZWKTEON-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204 °C (dec.)(lit.)
-
溶解度:H2O:50 mg/mL
-
稳定性/保质期:
在常温常压下稳定,应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):-0.39
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:75.7
-
氢给体数:4
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2922509090
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : DL-Normetanephrine hydrochloride
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 1011-74-1
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Skin irritation (Category 2), H315
Eye irritation (Category 2), H319
Specific target organ toxicity - single exposure (Category 3), H335
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Xi Irritant R36/37/38
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Warning
Hazard statement(s)
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H335 May cause respiratory irritation.
Precautionary statement(s)
P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
Safety data sheet available on request.
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
: DL-α-(Aminomethyl)-4-hydroxy-3-methoxybenzenemethanol
Synonyms
Formula : C9H13NO3 · HCl
Molecular Weight : 219,67 g/mol
CAS-No. : 1011-74-1
EC-No. : 213-787-2
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
(±)-α-(Aminomethyl)-4-hydroxy-3-methoxybenzyl alcohol hydrochloride
Skin Irrit. 2; Eye Irrit. 2; STOT -
SE 3; H315, H319, H335
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
(±)-α-(Aminomethyl)-4-hydroxy-3-methoxybenzyl alcohol hydrochloride
Xi, R36/37/38 -
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx), Hydrogen chloride gas
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end use(s)
A part from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Full contact
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: 480 min
Material tested:Dermatril® (KCL 740 / Z677272, Size M)
Splash contact
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: 480 min
Material tested:Dermatril® (KCL 740 / Z677272, Size M)
data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 87300, test method: EN374
If used in solution, or mixed with other substances, and under conditions which differ from EN 374,
contact the supplier of the CE approved gloves. This recommendation is advisory only and must
be evaluated by an industrial hygienist and safety officer familiar with the specific situation of
anticipated use by our customers. It should not be construed as offering an approval for any
specific use scenario.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 204 °C
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16: Other information
Full text of H-Statements referred to under sections 2 and 3.
Eye Irrit. Eye irritation
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H335 May cause respiratory irritation.
Skin Irrit. Skin irritation
STOT SE Specific target organ toxicity - single exposure
Full text of R-phrases referred to under sections 2 and 3
Xi Irritant
R36/37/38 Irritating to eyes, respiratory system and skin.
Further information
Copyright 2013 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
反应信息
-
作为反应物:描述:DL-去甲变肾上腺素盐酸盐 、 rhodamine 6G 在 N,N-二异丙基乙胺 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 96.0h, 生成 2-[3-(ethylamino)-6-ethylimino-2,7-dimethylxanthen-9-yl]-N-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)ethyl]benzamide参考文献:名称:Efficient synthesis of rhodamine conjugates through the 2′-position摘要:Reaction of substrates containing primary amines with rhodamine 2'-esters cleanly produces fluorescent rhodamine 2'-amide conjugates at ambient temperature. Only primary amines react with the esters under these conditions. Chemoselectivity can thus be achieved in substrates containing different types of amines (C) 2000 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0960-894x(00)00279-1
文献信息
-
Development of New HPLC Chiral Stationary Phases Based on Native and Derivatized Cyclofructans作者:Ping Sun、Chunlei Wang、Zachary S. Breitbach、Ying Zhang、Daniel W. ArmstrongDOI:10.1021/ac902257a日期:2009.12.15An unusual class of chiral selectors, cyclofructans, is introduced for the first time as bonded chiral stationary phases. Compared to native cyclofructans (CFs), which have rather limited capabilities as chiral selectors, aliphatic- and aromatic-functionalized CF6s possess unique and very different enantiomeric selectivities. Indeed, they are shown to separate a very broad range of racemic compounds. In particular, aliphatic-derivatized CF6s with a low substitution degree baseline separate all tested chiral primary amines. It appears that partial derivatization on the CF6 molecule disrupts the molecular internal hydrogen bonding, thereby making the core of the molecule more accessible. In contrast, highly aromatic-functionalized CF6 stationary phases lose most of the enantioselective capabilities toward primary amines, however they gain broad selectivity for most other types of analytes. This class of stationary phases also demonstrates high “loadability” and therefore has great potential for preparative separations. The variations in enantiomeric selectivity often can be correlated with distinct structural features of the selector. The separations occur predominantly in the presence of organic solvents.首次引入一类不寻常的手性选择剂——环糊精作为键合型手性固定相。与天然环糊精(CFs)相比,其作为手性选择剂的能力相当有限,而脂族和芳族功能化的CF6具有独特且截然不同的对映体选择性。事实上,它们能够分离非常广泛的消旋化合物。特别是,低取代度的脂族衍生化CF6能够基线分离所有测试的手性伯胺。似乎部分衍生化作用于CF6分子会破坏分子内部的氢键,从而使分子核心更易接近。相比之下,高度芳族功能化的CF6固定相在伯胺的对映选择能力方面丧失了大部分能力,但它们对大多数其他类型的分析物获得了广泛的选择性。这类固定相还表现出高“负荷能力”,因此具有在制备分离方面的巨大潜力。对映体选择性的变化通常可以与选择剂的独特结构特征相关联。分离主要在有机溶剂存在下发生。
-
Studies on radioimmunoassay of metanephrine.作者:AKIRA SHIRAHATA、MASANORI YOSHIOKA、MISAO MATSUSHITA、ZENZO TAMURADOI:10.1248/cpb.28.2994日期:——Antigens of 3-O-methylcatecholamines (metanephrine, normetanephrine, 3-methoxy-tyramine) were prepared, keeping the methoxyl and hydroxyl groups of the benzene ring and the side chain intact. Rabbits were immunized with the metanephrine antigen and sufficient antiserum to develop a specific radioimmunoassay of metanephrine was obtained. The detection limit of metanephrine in the radioimmunoassay was 0.03 pmol. The radioimmunoassay was useful for the measurement of urinary metanephrine and of rat liver catechol-O-methyltransferase activity.
-
Dopamine analog amide申请人:——公开号:US20010056116A1公开(公告)日:2001-12-27The invention involves the formation of a prodrug from a fatty acid carrier and a neuroactive drug. The prodrug is stable in the environment of both the stomach and the bloodstream and may be delivered by ingestion. The prodrug passes readily through the blood brain barrier. Once in the central nervous system, the prodrug is hydrolyzed into the fatty acid carrier and the drug to release the drug. In a preferred embodiment, the carrier is 4, 7, 10, 13, 16, 19 docosahexa-enoic acid and the drug is dopamine. Both are normal components of the central nervous system. The covalent bond between the drug and the carrier preferably is an amide bond, which bond may survive the conditions in the stomach. Thus, the prodrug may be ingested and will not be hydrolyzed completely into the carrier molecule and drug molecule in the stomach.
-
Thiazole derivatives as phosphodiesterase iv inhibitors申请人:Eggenweiler Hans-Michael公开号:US20050222160A1公开(公告)日:2005-10-06Thiazole derivatives of the formula I in which R 1 , R 2 , R 3 , V, W, X and B are as defined in claim 1, act as phosphodiesterase IV inhibitors and can be employed for the treatment of osteoporosis, tumours, cachexia, atherosclerosis, rheumatoid arthritis, multiple sclerosis, diabetes mellitus, inflammatory processes, allergies, asthma, autoimmune diseases, myocardial diseases and AIDS.
-
Pyrrolopyrimidines as phosphodiesterase vII inhibitors申请人:Eggenweiler Hans-Michael公开号:US20050059686A1公开(公告)日:2005-03-17Pyrrolopyrimidine derivatives of the formula I in which R 3 , R 4 , R 5 , R 6 and X are as defined in claim 1, act as phosphodiesterase VII inhibitors and can be employed for the treatment of osteoporosis, tumours, cachexia, atherosclerosis, rheumatoid arthritis, multiple sclerosis, diabetes mellitus, inflammatory processes, allergies, asthma, autoimmune diseases, myocardial diseases and AIDS.
表征谱图
-
氢谱1HNMR
-
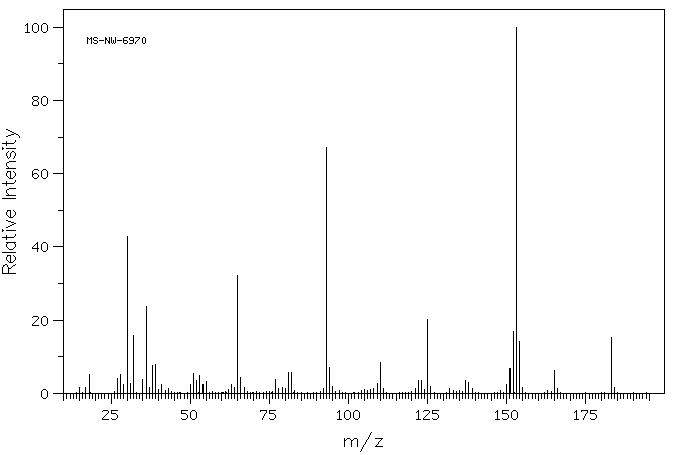
质谱MS
-
碳谱13CNMR
-
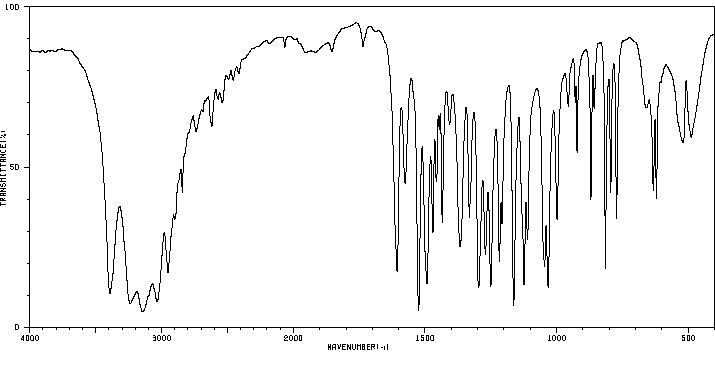
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚