tert-butyl 3-methyl-2-(4-methylphenylsulfonamido)butanoate | 60522-08-9
中文名称
——
中文别名
——
英文名称
tert-butyl 3-methyl-2-(4-methylphenylsulfonamido)butanoate
英文别名
N-p-Toluenesulfonyl-l-valine tert-butyl ester;tert-butyl (2S)-3-methyl-2-[(4-methylphenyl)sulfonylamino]butanoate
CAS
60522-08-9
化学式
C16H25NO4S
mdl
——
分子量
327.445
InChiKey
PISNTUWFJNPSIT-AWEZNQCLSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:427.0±55.0 °C(Predicted)
-
密度:1.118±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:22
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.56
-
拓扑面积:80.8
-
氢给体数:1
-
氢受体数:5
上下游信息
反应信息
-
作为反应物:参考文献:名称:Hlavacek,J. et al., Collection of Czechoslovak Chemical Communications, 1976, vol. 41, p. 2079 - 2087摘要:DOI:
-
作为产物:参考文献:名称:克服脱芳基化问题:芳烷基烯丙基醚,胺和氨基酸的钯(II)催化的化学,区域和立体选择性烯丙基氧化。摘要:我们在此报告了芳基烯丙基醚,胺和氨基酸的Pd(II)/双亚砜催化的分子内烯丙基CH乙酰氧基化,同时保留了不稳定的烯丙基部分。从机理上讲,反应是通过从烯丙基位置到乙烯基位置的独特的双键异构化进行的,然后进行分子内羧基palpalpalation和β-氢化物消除途径。首次建立了具有出色的非对映选择性的N-烯丙基保护氨基酸的C–H氧化,可通过1,3-syn加成提供五元杂环。DOI:10.1021/acs.orglett.0c02465
文献信息
-
Intramolecular NC rearrangements involving sulfonamide protecting groups作者:Amie Saidykhan、Richard D. Bowen、Richard T. Gallagher、William H.C. MartinDOI:10.1016/j.tetlet.2014.10.041日期:2015.1The reaction of amine derivatives orthogonally protected with an aryl sulfonamide and a carbamate, via a base-mediated nitrogen to carbon rearrangement is reported. This noteworthy isomerisation has implications for the use of sulfonamide protecting groups in synthesis.
-
Synthesis of (<i>Z</i>)-β-Chloro-enamides via a Base-Promoted <i>trans</i>-Hydroamidation of Alkynyl Chlorides Using 1,1-Dichloroalkenes as Precursor作者:Zhenming Zhang、Bei Fu、Xiaoshuo Wang、Zhipeng Hu、Xuanling Liu、Tao Wang、Junfeng ZhaoDOI:10.1021/acs.joc.2c00581日期:2022.7.1An efficient and general base-promoted reaction of 1,1-dichloroalkenes with secondary sulfonamides and amides for the synthesis of (Z)-β-chloro-enamides has been described. This reaction exhibits functional group tolerance under simple and mild conditions. Mechanistic study indicated that a stereoselective trans-hydroamidation of alkynyl chlorides generated in situ from 1,1-dichloroalkenes was the
-
Hlavacek, Jan; Fric, Ivo; Budesinsky, Milos, Collection of Czechoslovak Chemical Communications, 1988, vol. 53, # 11A, p. 2473 - 2494作者:Hlavacek, Jan、Fric, Ivo、Budesinsky, Milos、Blaha, KarelDOI:——日期:——
-
Synthesis of an array of potential matrix metalloproteinase inhibitors using a sequence of polymer-supported reagents作者:Marina Caldarelli、Jörg Habermann、Steven V. LeyDOI:10.1016/s0960-894x(99)00311-x日期:1999.7Polymer-supported reagents and sequestering agents may be used to generate an array of variously substituted hydroxamic acid derivatives as potential inhibitors of matrix metalloproteinases without any chromatographic purification step. (C) 1999 Elsevier Science Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
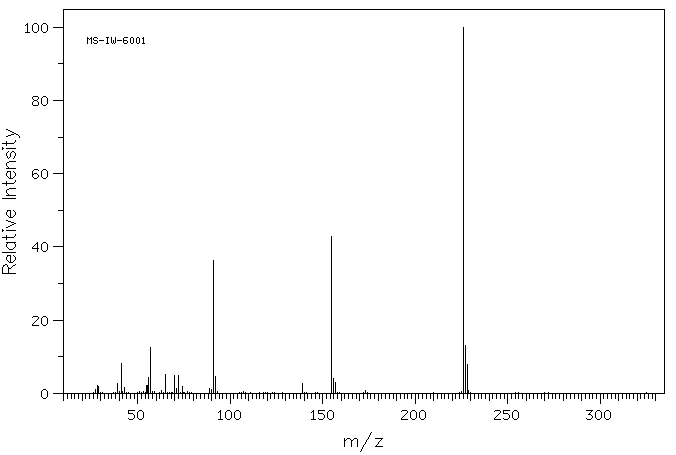
质谱MS
-
碳谱13CNMR
-
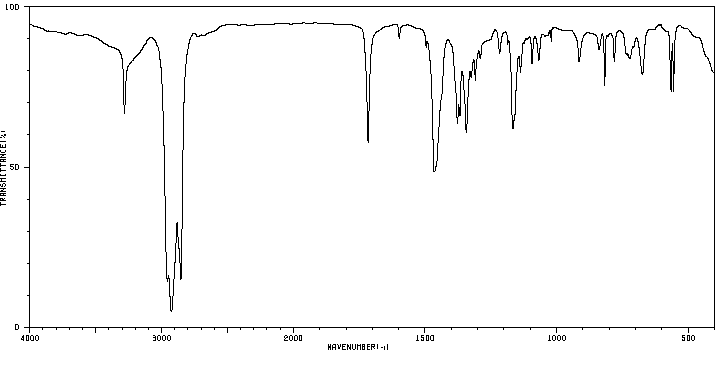
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸