2-(4-联苯基)-5-苯基恶二唑 | 852-38-0
中文名称
2-(4-联苯基)-5-苯基恶二唑
中文别名
2-(4-联苯基)-5-苯基-1,3,4-恶二唑;2-(4-联苯基)-5-苯基-1,3,4-噁二唑;2-(1,1"-联苯-4-基)-5-苯基-1,3,4-恶二唑
英文名称
2-biphenyl-4-yl-5-phenyl-[1,3,4]oxadiazole
英文别名
PBD;2-phenyl-5(4-biphenylyl)-1,3,4-oxadiazole;2-Phenyl-5-(4-biphenylyl)-1,3,4-oxadiazol;2-(4-biphenylyl)-5-phenyl-1,3,4-oxadiazole;Phenyl-biphenylyl-oxadiazol;2-Phenyl-5-biphenyl-4-yl-<1,3,4>-oxadiazol;2-Phenyl-5-<4-biphenylyl>-1,3,4-oxadiazol;2-Phenyl-5-biphenylyl-(4)-1,3,4-oxdiazol;2-Phenyl-5-biphenyl-oxadiazol;Phenyl-biphenyl-oxadiazol;2-Phenyl-5-biphenyl<4>yl-<1,3,4>oxadiazol;2-Phenyl-5-(4'-biphenyl)-1,3,4-oxadiazol;2-phenyl-5-(4-phenylphenyl)-1,3,4-oxadiazole
CAS
852-38-0
化学式
C20H14N2O
mdl
MFCD00003100
分子量
298.344
InChiKey
WMAXWOOEPJQXEB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:167-169 °C(lit.)
-
沸点:439.76°C (rough estimate)
-
密度:1.1743 (rough estimate)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:23
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:38.9
-
氢给体数:0
-
氢受体数:3
安全信息
-
安全说明:S22,S24/25
-
WGK Germany:3
-
海关编码:2934999090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
| Name: | Pbd Scintillation Grade Material Safety Data Sheet |
| Synonym: | 1,3,4-Oxodiazole,2-1,1'-Biphenyl-4-yl-5-Pheny |
| CAS: | 852-38-0 |
Synonym:1,3,4-Oxodiazole,2-1,1'-Biphenyl-4-yl-5-Pheny
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 852-38-0 | 1,3,4-Oxadiazole, 2-1,1'-Biphenyl-4-yl | ca 100 | 212-712-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 852-38-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 168.00 - 170.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C20H14N2O
Molecular Weight: 298.33
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 852-38-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,3,4-Oxadiazole, 2-1,1'-Biphenyl-4-yl-5-Phenyl - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 852-38-0: No information available.
Canada
CAS# 852-38-0 is listed on Canada's NDSL List.
CAS# 852-38-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 852-38-0 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:适合用作激光染料。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(4'-vinylbiphenyl-4-yl)-5-phenyl-1,3,4-oxadiazole 136180-42-2 C22H16N2O 324.382
反应信息
-
作为反应物:描述:参考文献:名称:Kurfuerst, Antonin; Lhotak, Pavel; Nadenik, Petr, Collection of Czechoslovak Chemical Communications, 1991, vol. 56, # 7, p. 1495 - 1504摘要:DOI:
-
作为产物:描述:参考文献:名称:取代的1,3,4-恶二唑的17 O NMR研究。摘要:通过(17)O NMR光谱研究了三个系列的取代的1,3,4-恶二唑。化学位移值与经验的哈米特参数以及计算的键长和化学屏蔽值相关。DOI:10.1002/mrc.2804
文献信息
-
Selective Methylation of Arenes: A Radical C−H Functionalization/Cross‐Coupling Sequence作者:Fabien Serpier、Fei Pan、Won Seok Ham、Jérôme Jacq、Christophe Genicot、Tobias RitterDOI:10.1002/anie.201804628日期:2018.8.13A selective, nonchelation‐assisted methylation of arenes has been developed. The overall transformation, which combines a C−H functionalization reaction with a nickel‐catalyzed cross‐coupling, offers rapid access to methylated arenes with high para selectivity. The reaction is amenable to late‐stage methylation of small‐molecule pharmaceuticals.
-
Palladium-catalyzed one-pot synthesis of diazoles via tert-butyl isocyanide insertion作者:Xiang-Yuan Fan、Xiao Jiang、Ying Zhang、Zhen-Bang Chen、Yong-Ming ZhuDOI:10.1039/c5ob01328c日期:——
An efficient one-pot palladium-catalyzed reaction for the synthesis of diazoles from readily available hydrazides and aryl halide
via isocyanide insertion/cyclization sequence has been developed. -
ORGANIC OPTOELECTRONIC DEVICE AND DISPLAY DEVICE申请人:SAMSUNG SDI CO., LTD.公开号:US20170222158A1公开(公告)日:2017-08-03An organic optoelectronic device and a display device including the same, the organic optoelectronic device including: an anode and a cathode facing each other; a light-emitting layer positioned between the anode and the cathode; a hole transport layer positioned between the anode and the light-emitting layer; an auxiliary hole transport layer positioned between the hole transport layer and the light-emitting layer; an electron transport layer positioned between the cathode and the light-emitting layer; and an auxiliary electron transport layer positioned between the electron transport layer and the light-emitting layer, wherein the auxiliary electron transport layer includes at least one kind of first compound represented by chemical formula 1, and the auxiliary hole transport layer includes at least one kind of second compound represented by a combination of a moiety represented by chemical formula 2, a moiety represented by chemical formula 3 and a moiety represented by chemical formula 4.
-
Synthesis of Luminophores Based on Thiazole Derivatives of PBD作者:Pavel Lhoták、Antonín KurfürstDOI:10.1135/cccc19931898日期:——
Organic luminophore 2-(biphenyl-4-yl)-5-phenyl-1,3,4-oxadiazole (PBD,
III ) was converted by series of reaction into thioamideVIII which cyclized with substituted bromoacetylarenesX to give the bifluorophore systemXI . The thiazole analogXIII was obtained by analogous reaction of bromoacetyl derivativeV with thiobenzamide. All the thus-prepared compounds exhibit pronounced fluorescence in solution as well as in the crystalline state. -
A simple and efficient one step synthesis of 1,3,4-oxadiazoles utilizing polymer-supported reagents and microwave heating作者:Ying Wang、Daryl R. Sauer、Stevan W. DjuricDOI:10.1016/j.tetlet.2005.10.131日期:2006.11,3,4-Oxadiazoles can be rapidly and efficiently synthesized from a variety of carboxylic acids and acid hydrazides in one simple step. The use of commercially available PS-PPh3 resin combined with microwave heating delivered the product 1,3,4-oxadiazoles in high yields and purities.
表征谱图
-
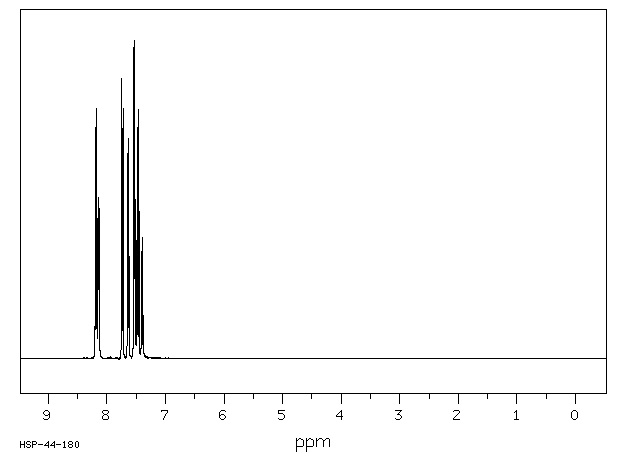
氢谱1HNMR
-
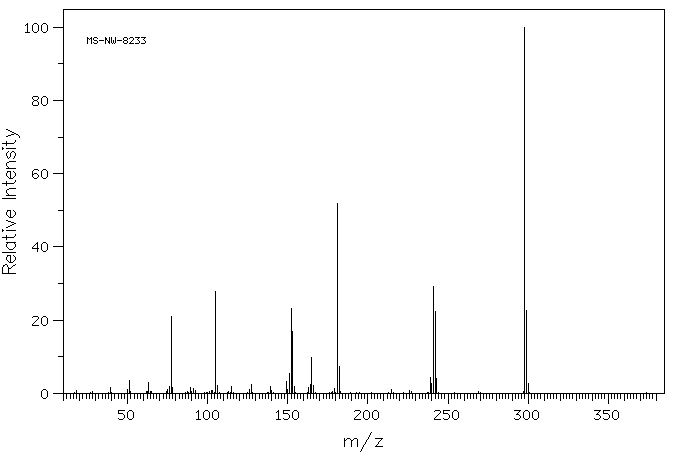
质谱MS
-
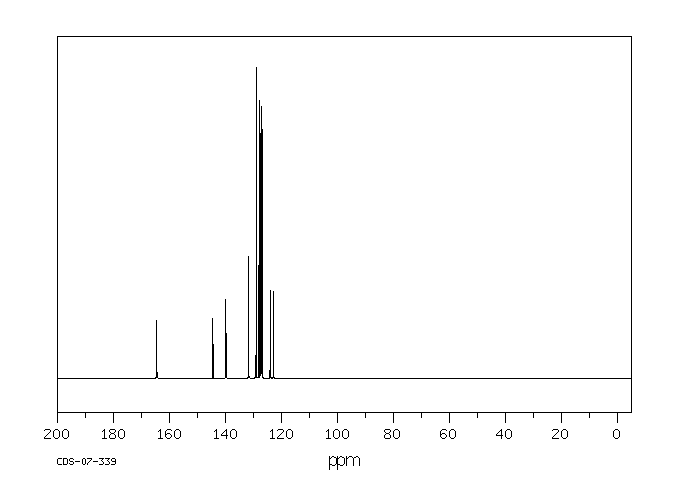
碳谱13CNMR
-
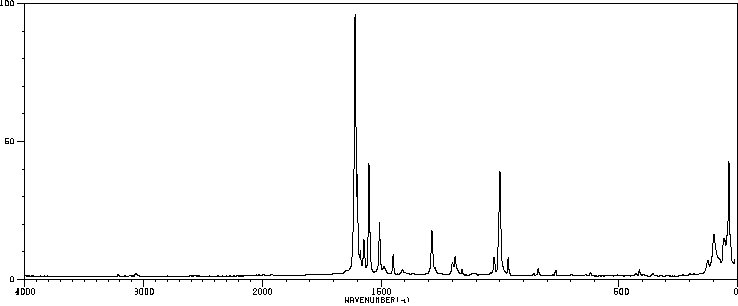
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫