1-乙基-4-[4-(4-乙基苯基)丁-1,3-二炔基]苯 | 35672-48-1
中文名称
1-乙基-4-[4-(4-乙基苯基)丁-1,3-二炔基]苯
中文别名
——
英文名称
1,4-bis(p-ethylphenyl)buta-1,3-diyne
英文别名
1,4-bis(4-ethylphenyl)buta-1,3-diyne;bis(4-ethylphenyl)butadiyne;1,4-bis(4-ethylphenyl)-1,3-butadiyne;Benzene, 1,1'-(1,3-butadiyne-1,4-diyl)bis[4-ethyl-;1-ethyl-4-[4-(4-ethylphenyl)buta-1,3-diynyl]benzene
CAS
35672-48-1
化学式
C20H18
mdl
——
分子量
258.363
InChiKey
YZJIXESJJUPTHY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:72 °C
-
沸点:387.9±35.0 °C(Predicted)
-
密度:1.04±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5.9
-
重原子数:20
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
反应信息
-
作为反应物:描述:1-乙基-4-[4-(4-乙基苯基)丁-1,3-二炔基]苯 在 水 、 二甲基亚砜 、 potassium hydroxide 作用下, 反应 4.0h, 以90%的产率得到2,5-bis(4-ethylphenyl)furan参考文献:名称:芳基化呋喃,吡咯和噻吩的通用方法摘要:已经开发了用于芳基取代的五元杂环的通用且实用的合成方法。在KOH(30%)存在下,1,4-二芳基-1,3-丁二炔与水,伯胺和Na 2 S·9H 2 O在DMSO中于80°C进行环缩合反应,得到2,5 -二芳基呋喃,1,2,5-三取代的吡咯和2,5-二芳基噻吩的产率高至高。进一步的研究表明,芳烃取代的五元杂环还可以通过一锅,两步策略由DMSO中的末端炔烃首先由CuCl催化,然后通过添加KOH促进1的环缩合而合成。原位生成3-丁二炔。DOI:10.1016/j.tet.2014.09.025
-
作为产物:参考文献:名称:Cu(II)-CMC:用于氧化炔均偶联反应的温和、高效且可回收的催化剂摘要:摘要 在羧甲基纤维素钠 (Na-CMC) 上异质化的 Cu(II) 已通过不同的技术进行了彻底的表征。Cu(II)-CMC首次应用于多种末端炔烃的均偶联反应。该催化剂提供了良好到极好的所需产物的产率,并且可以重复使用六次而不会损失催化活性。Cu(II)-CMC 催化方案是在温和条件下合成 1,3-二炔的一种新的有效途径。DOI:10.1515/znb-2017-0009
文献信息
-
Copper-Mediated Deacylative Coupling of Ynones via C–C Bond Activation under Mild Conditions作者:Lili Feng、Tingjun Hu、Saisai Zhang、Heng-Ying Xiong、Guangwu ZhangDOI:10.1021/acs.orglett.9b03684日期:2019.12.6The intermolecular deacylative coupling of unstrained ynones via C-C bond activation was accomplished by a CuCl-bpy system under mild reaction conditions. This protocol features facile cleavage of the C-C bond at room temperature, broad substrate scope, and efficient construction of important symmetric and unsymmetrical 1,3-diyne adducts through homo or cross coupling of ynones, respectively. The preliminary通过温和的反应条件下的CuCl-bpy系统完成了未应变的炔酮通过CC键活化的分子间脱酰基反应。该协议的特点是在室温下容易裂解CC键,广泛的底物范围,以及通过炔酮的均相或交联分别有效构建重要的对称和不对称1,3-二炔加合物的方法。初步的机械研究表明,此过程可能涉及酰基铜(III)配合物。
-
Cobalt‐Catalyzed Temperature‐Dependent Annulation of 3‐Aryl‐1,2,4‐oxadiazolones with 1,3‐Diynes: An Approach to π‐Conjugated Molecules作者:Wenge Zhang、Hongji Li、Lei WangDOI:10.1002/adsc.201801165日期:2019.6.18A Cobalt‐catalyzed annulation of 3‐aryl‐1,2,4‐oxadiazolones with 1,3‐diynes has been developed, in which reaction temperature variation considerably affects the cyclization orientation and afforded structurely distinctive products in good yields and high regioselectivity. Additionally, the late‐stage transformation of the cyclization products into N‐containing heterocycles is also demonstrated.
-
Cobalt(<scp>ii</scp>)-catalyzed hydroarylation of 1,3-diynes and internal alkynes with picolinamides promoted by alcohol作者:Yuan Gao、Mengfan Zhang、Chaoyu Wang、Zhen Yang、Xianqiang Huang、Ruokun Feng、Chenze QiDOI:10.1039/d0cc05616b日期:——The Co(II)-catalyzed selective C–H alkenylation of picolinamides with 1,3-diynes has been developed. This protocol can be applied to a variety of 1,3-diynes. In addition, both symmetrical and unsymmetrical internal alkynes were well tolerated, affording the corresponding alkenyl arenes. Moreover, control experiments indicated that C–H bond cleavage may be involved in the rate-determining step. Furthermore
-
Copper(<scp>i</scp>) chloride catalysed room temperature C<sub>sp</sub>–C<sub>sp</sub> homocoupling of terminal alkynes mediated by visible light作者:Arunachalam Sagadevan、Vaibhav Pramod Charpe、Kuo Chu HwangDOI:10.1039/c6cy01400c日期:——We developed a technique mediated by visible light for the aerobic homocoupling of terminal alkynes to synthesize 1,3-conjugated diynes using a copper(I) chloride catalyst at room temperature. Compared with previously reported thermal processes, this photochemical method is simple, uses only mild reaction conditions, produces high yields and works well for substrates with electron-withdrawing groups
-
Practical Oxidative Homo- and Heterocoupling of Terminal Alkynes Catalyzed by Immobilized Copper in MCM-41作者:Ruian Xiao、Ruiya Yao、Mingzhong CaiDOI:10.1002/ejoc.201200359日期:2012.8A practical oxidative homo- and heterocoupling of terminal alkynes was achieved in CH2Cl2 at 25 °C by using a 3-(2-aminoethylamino)propyl-functionalized MCM-41-immobilized copper(I) complex (MCM-41–2N-CuI, 1 mol-%) as the catalyst, piperidine (0.1 or 3 equiv.) as the base, and air as the environmentally friendly co-oxidant, yielding a variety of symmetrical and unsymmetrical 1,4-disubstituted 1,3-diynes
表征谱图
-
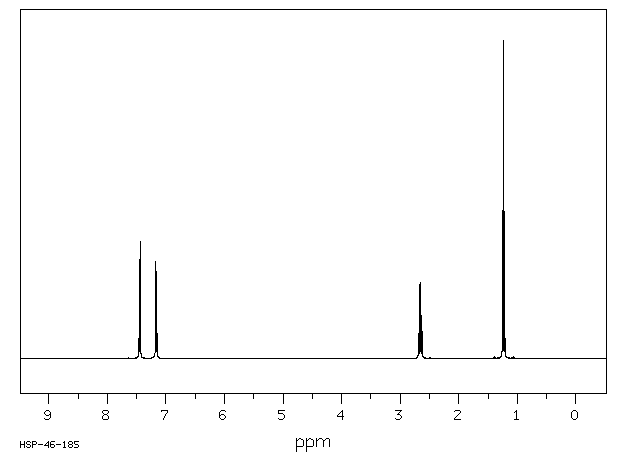
氢谱1HNMR
-
质谱MS
-
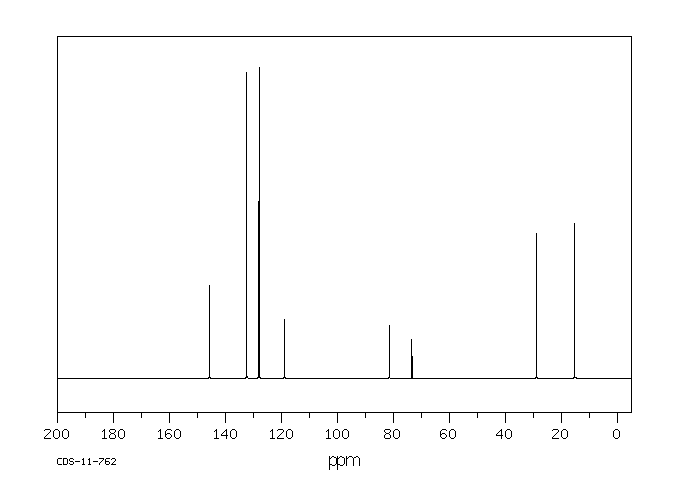
碳谱13CNMR
-
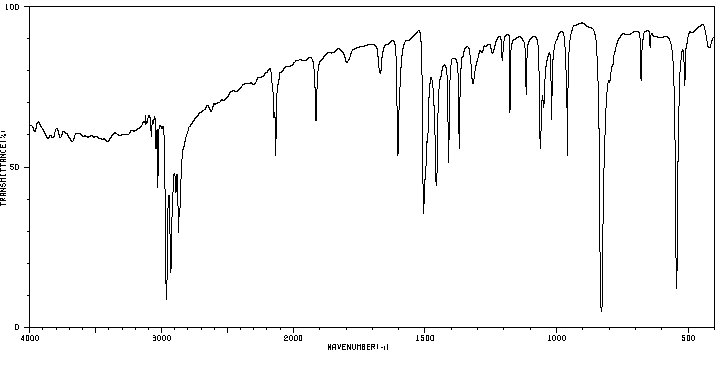
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫