2-甲基-6-甲氧基苯-1,4-二醇 | 28814-66-6
中文名称
2-甲基-6-甲氧基苯-1,4-二醇
中文别名
——
英文名称
2-methoxy-6-methylbenzene-1,4-diol
英文别名
2-methyl-6-methoxybenzene-1,4-diol;2-methoxy-6-methyl-hydroquinone;2,5-dihydroxy-3-methoxytoluene;3-Methoxytoluhydroquinone;3-methoxy-2,5-toluquinol;2-Methoxy-6-methyl-hydrochinon;1,4-Dihydroxy-2-methoxy-6-methylbenzene
CAS
28814-66-6
化学式
C8H10O3
mdl
——
分子量
154.166
InChiKey
RNDWEMBYMPZABZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:321.3±37.0 °C(Predicted)
-
密度:1.217±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:49.7
-
氢给体数:2
-
氢受体数:3
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基-6-甲基苯酚 2-methyl-6-methoxyphenol 2896-67-5 C8H10O2 138.166 邻香草醛 3-methoxy-2-hydroxybenzaldehyde 148-53-8 C8H8O3 152.15 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,2,5-三甲氧基-3-甲基苯 1,2,5-trimethoxy-3-methylbenzene 38790-14-6 C10H14O3 182.219 6-甲基-1,2,4-苯三醇 2,3,5-trihydroxytoluene 767-81-7 C7H8O3 140.139 —— 3,6-dihydroxy-4-methoxy-2-methyl-benzaldehyde 872304-60-4 C9H10O4 182.176
反应信息
-
作为反应物:描述:参考文献:名称:Kusaka, Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan, 1941, vol. 61, p. 355,364摘要:DOI:
-
作为产物:描述:邻香草醛 在 palladium on activated charcoal potassium dihydrogenphosphate 、 titanium(III) chloride 、 potassium nitrososulfonate 、 氢气 、 溶剂黄146 作用下, 以 乙醚 、 乙酸乙酯 、 丙酮 为溶剂, 反应 73.17h, 生成 2-甲基-6-甲氧基苯-1,4-二醇参考文献:名称:DNA cleavage by di- and trihydroxyalkylbenzenes. Characterization of products and the roles of O2, Cu(II), and alkali摘要:Several 5-alkyl-1,3-dihydroxybenzene (5-alkylresorcinol, 1) and 6-alkyl-1,2,4-trihydroxybenzene (2) derivatives were prepared and used to study the mechanism by which such compounds effect Cu(II)-dependent DNA strand scission. Comparison of the methyl, n-pentyl, n-undecyl, and n-hexadecyl derivatives in each structural series indicated that the efficiency of DNA cleavage increased with increasing length of the alkyl substituent. DNA cleavage by the 5-alkylresorcinols appears to involve initial oxygenation of the benzene nucleus, a process that occurs readily at alkaline pH in the presence of Cu2+ and O-2. The resulting trihydroxylated benzenes mediate DNA cleavage in a reaction dependent on the presence of both Cu2+ and O-2. The mechanism appears to involve reduction of CU2+ by the trihydroxybenzene moiety in 2, with subsequent formation of reactive oxygen species. The ability of catalase and dimethyl sulfoxide to suppress DNA strand scission is consistent with the intermediacy of H2O2 and (OH)-O-. in the DNA strand scission process.DOI:10.1021/ja00156a005
文献信息
-
First total synthesis of antrocamphin A and its analogs as anti-inflammatory and anti-platelet aggregation agents作者:Chia-Lin Lee、Chi-Huan Huang、Hui-Chun Wang、Da-Wei Chuang、Ming-Jung Wu、Sheng-Yang Wang、Tsong-Long Hwang、Chin-Chung Wu、Yeh-Long Chen、Fang-Rong Chang、Yang-Chang WuDOI:10.1039/c0ob00616e日期:——Naturally occurring antrocamphin A (1) is a potent anti-inflammatory compound from the edible fungus Antrodia camphorata (Taiwanofungus camphoratus), whose wild fruiting body is used as a valuable folk medicine in Taiwan. This study is the first total synthesis of antrocamphin A (1) and its analogs. Their inhibition ability on NO release, superoxide anion generation, elastase release and platelet aggregation are reported herein.
-
Dual Behavior of Masked <i>o</i>-Benzoquinones in Intramolecular Diels−Alder Reactions. Expedient Synthesis of Highly Functionalized <i>cis</i>-Decalins from 2-Methoxyphenols作者:Po-Yi Hsu、Rama Krishna Peddinti、Santhosh Kumar Chittimalla、Chun-Chen LiaoDOI:10.1021/jo0510179日期:2005.11.1added 2,4-dienol. The majority of the cycloadducts resulted from the diene property of MOBs in intramolecular Diels−Alder reactions smoothly underwent Cope rearrangement to furnish cis-decalins as sole products in excellent to quantitative yields that provides a short and efficient entry to polyfunctionized cis-decalins from 2-methoxyphenols. Furthermore, the variation of dienophilic and diene characters潜在的双重行为作为掩蔽的二烯部分的二烯和亲双烯体ö -benzoquinones(MOBS)10A - ë - 12A - ë,在2-甲氧基苯酚氧化产生1 - 3中的适当dienols存在下与BTIB,在其分子内Diels-Alder(IMDA)反应已经过检查。MOB 10a - d,11a,b,d和12a,b,d的IMDA反应导致高度官能化的草三环化合物18a - d,图19A,b,d,和20A,b,d分别与伴随形成的顺式-decalin衍生物21a的- d,22A,b,d,和23A,b,d的高度区域选择性和立体选择性的方式。但是,MOB 10e - 12e仅提供草三环化合物18e - 20e。顺式的形成这些IMDA反应中的十氢化萘除了具有二烯的作用外,还表明了MOB的亲二烯性。由于MOB的双重特征,在每个反应中获得的两个环加合物的比例取决于环己二烯酮部分和所添加的2,4-二烯醇两者上的
-
COGNITIVE IMPAIRMENT AMELIORANT申请人:Yamaguchi Kikuji公开号:US20110287106A1公开(公告)日:2011-11-24A cognitive impairment ameliorant is provided that is useful for the prevention or treatment of cognitive impairment, and particularly Alzheimer's disease. The cognitive impairment ameliorant contains royal jelly as an active ingredient thereof.提供一种认知障碍改善剂,可用于预防或治疗认知障碍,特别是阿尔茨海默病。该认知障碍改善剂包含蜂王浆作为其活性成分。
-
E-Dakin reaction: oxidation of hydroxybenzaldehydes to phenols with electrochemically generated peroxodicarbonate as sustainable ex-cell oxidizer作者:Fiona Sprang、Niclas Schupp、Philipp J. Kohlpaintner、Lukas J. Gooßen、Siegfried R. WaldvogelDOI:10.1039/d3gc04597h日期:——Electrochemically generated peroxodicarbonate solution efficiently oxidizes hydroxybenzaldehydes to valuable phenols. The reaction is carried out in aqueous media without additional solvents or additives. The reaction gives high yields of up to 97% and tolerates a broad variety of substituents, as demonstrated by 20 diverse examples. It was successfully scaled to multi-gram range. This transformation
-
Experimental study of the generation of periodic internal waves by the boundary layer on a rotating disk作者:Yu. D. Chashechkin、Yu. V. Kistovich、Yu. S. Il'inykhDOI:10.1134/1.1333872日期:2000.11
表征谱图
-
氢谱1HNMR
-
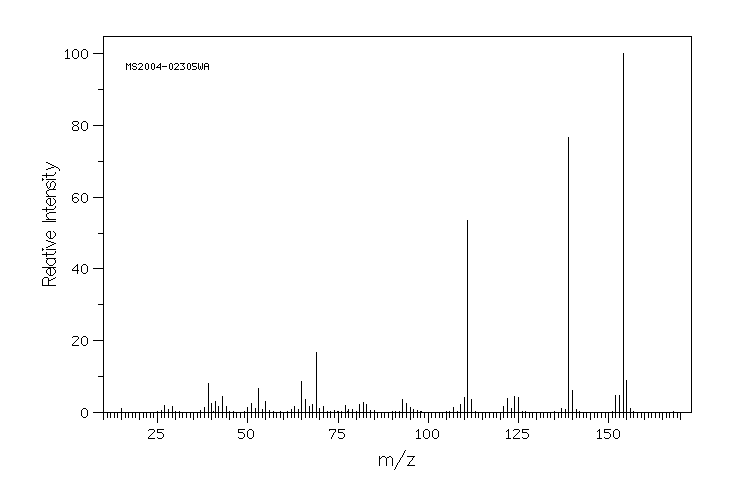
质谱MS
-
碳谱13CNMR
-
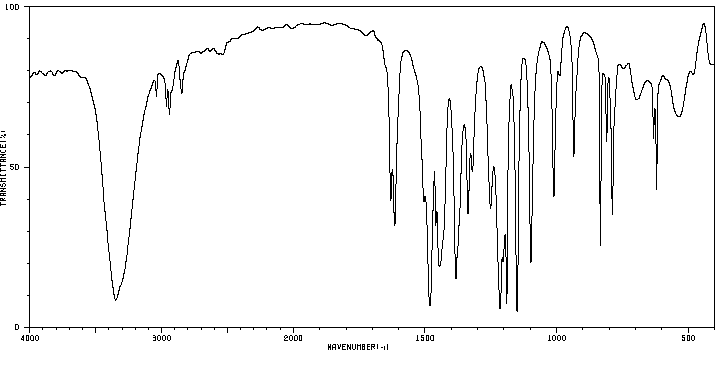
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚