二氢-6-甲基-2,4-二嘧啶酮 | 2434-49-3
中文名称
二氢-6-甲基-2,4-二嘧啶酮
中文别名
5,6-二氢-6-甲基尿嘧啶
英文名称
5,6-Dihydro-6-methyluracil
英文别名
5,6-dihydro-6-methyl-2,4-(1H,3H)-pyrimidinedione;6-methyl-(R,S)-dihydrouracil;6-methyl-dihydrouracil;6-methyldihydrouracil;6-methyl-dihydro-pyrimidine-2,4-dione;6-methyl-1,3-diazinane-2,4-dione
CAS
2434-49-3
化学式
C5H8N2O2
mdl
——
分子量
128.131
InChiKey
XQLIRTZXJDEQAO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:218-220°C
-
密度:1.149±0.06 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化物直接接触。
计算性质
-
辛醇/水分配系数(LogP):-0.7
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:58.2
-
氢给体数:2
-
氢受体数:2
安全信息
-
安全说明:S22,S24/25
-
海关编码:2933599090
SDS
上下游信息
反应信息
-
作为反应物:描述:二氢-6-甲基-2,4-二嘧啶酮 在 磷酸 、 dihydropyrimidinase 、 N-carbamoyl- -alanine amidohydrolase 、 nickel dichloride 作用下, 以 aq. phosphate buffer 为溶剂, 生成 DL-3-氨基-N-丁酸参考文献:名称:从 5- 和 6- 单取代二氢尿嘧啶开始生产富含对映体的 β-丙氨酸衍生物的新生物催化路线摘要:摘要 利用嘧啶分解代谢酶(二氢嘧啶酶 (EC 3.5.2.2)、N-氨基甲酰基-β-丙氨酸酰胺水解酶 (EC 3.5.1.6))的催化混杂性,从 5- 和6-单取代的二氢尿嘧啶已使用模拟方法进行了评估。在这项工作中,已经显示了苜蓿中华根瘤菌的二氢嘧啶酶对 6-单取代的二氢尿嘧啶衍生物的 S-对映选择性特征。已经证明来自根癌农杆菌的 N-氨基甲酰基-β-丙氨酸酰胺水解酶对两种不同的 N-氨基甲酰基-β-氨基酸具有反向的 R-/S-对映选择性。DOI:10.1016/j.procbio.2012.07.026
-
作为产物:描述:N1,N3-dibenzyloxymethyl-6-methyl-5,6-dihydrouracil 在 三氟乙酸 作用下, 反应 7.0h, 以30%的产率得到二氢-6-甲基-2,4-二嘧啶酮参考文献:名称:DeFrees, Shawn A.; Reddy, Kalakota S.; Cassady, John M., Synthetic Communications, 1988, vol. 18, # 2, p. 213 - 220摘要:DOI:
文献信息
-
Modified multicomponent Biginelli–Atwal reaction towards a straightforward construction of 5,6-dihydropyrimidin-4-ones作者:Etienne Pair、Vincent Levacher、Jean-François BrièreDOI:10.1039/c5ra08792a日期:——
A modified multicomponent Biginelli condensation with Meldrum's acid affords a straightforward access to 5,6-dihydropyrimidin-4-ones through a domino Knoevenagel-aza-Michael-Cyclocondensation (KaMC) reaction.
使用Meldrum's酸对多组分Biginelli缩合进行改进,通过多米诺Knoevenagel-aza-Michael-环缩合(KaMC)反应,可以简单直接地获得5,6-二氢吡啶-4-酮。 -
Synthesis of 2- (and 6-) -dithian-2-yluracil nucleosides and their conversion into nucleoside derivatives作者:A. Rosenthal、Robert H. DoddDOI:10.1016/s0008-6215(00)83658-7日期:1980.1)pyrimidinone ( 3 ), O 2 ,2′-anhydro-5,6-di-hydro-6-( S )-(1,3-dithian-2-yl)-5′- O -trityluridine ( 4 ), and 2-(1,4-dihydroxybutyl)-1,3-dithiane ( 5 ) in yields of 15, 30, and 10% respectively. The structure of 3 was proved by its hydrolysis in acid to give 2-(1,3-dithian-2-yl)-4-pyrimidinone ( 6 ) and arabinose, and by desulfurization with Raney nickel to yield the known 2-methyl-1-(5- O -trityl-β- D -将2,2'-脱水-[1-(3-O-乙酰基-5-O-三苯甲基-β-D-阿拉伯呋喃糖基)尿嘧啶](1)添加至过量的2-litho-1,3-dithiane(2 )在-78°的环戊烷中得到2-(1,3-二噻吩-2-基)-1-(5-O-三苯甲基-β-D-阿拉伯呋喃糖基)-4(1 H)嘧啶酮(3),O 2 ,2'-脱水-5,6-二氢-6-(S)-(1,3-二硫-2-基)-5'-O-三苯甲基吡啶(4)和2-(1,4-二羟基丁基)-1,3-二噻吩(5)的产率分别为15%,30%和10%。3的结构通过其在酸中的水解得到2-(1,3-dithian-2-yl)-4-pyrimidinone(6)和阿拉伯糖,并通过阮内镍脱硫得到已知的2-甲基-甲基,从而证明了3的结构。 1-(5-O-三苯甲基-β-D-阿拉伯呋喃糖基)-4(1 H)-嘧啶酮(7)。不进行糖苷裂解的3的去三苯甲基化只能通过事先乙酰化为1-(2,
-
Linear free energy–steric strain energy relationships for the gem-di-methyl effect. Acid-catalysed ring closure of methyl-substituted 3-ureidopropionic acids作者:Iva B. Blagoeva、Bogdan J. Kurtev、Ivan G. PojarlieffDOI:10.1039/p29790001115日期:——statistical criteria. Linear relationships were obtained for the free energies in the reaction series for rates and equilibria exhibiting the gem-dimethyl effect with steric strain energies estimated from enthalpies of formation of homomorphic hydrocarbons. These estimates were obtained by means of Istomin and Palm's procedure and are of two kinds, the P values measuring the total strain energy and ΔP values根据Exner的统计标准,八个甲基取代的3-脲基丙酸的酸催化的闭环速率数据构成了等速动力学级数。对于速率和平衡,反应系列中的自由能获得线性关系,平衡率显示出宝石-二甲基效应,而同构碳氢化合物的生成焓估计其空间应变能。这些估计值是通过Istomin和Palm的方法获得的,有两种,P值测量总应变能,ΔP值测量模型烃中两个片段之间的应变。一些与ΔP相关的反应级数当官能团之一被视为恒定片段而分子的其余部分被视为可变取代基时,其值是0。其他反应系列与P值相关。该方法的优点在于,它直接考虑了无光泽相互作用的非可加性,并允许以单个相关性评估链上各个位置上的取代基的数据。为了环化成五元和六元环,当母体化合物的速率值接近时,数据形成单个反应系列。只有某些碳氢化合物模型和断裂方式才能获得令人满意的相关性。这些以及与P或与ΔP的相关性值,或是否可以完全获得相关性,表明反应的空间要求及其机理。对于速率加速度归因于
-
Kinetic Studies on Dehydrogenation Reaction of 5,6-Dihydro-2,4(1<i>H</i>,3<i>H</i>)-pyrimidinediones in Aqueous Solution Induced by Argon Arc Plasma or Hydrogen–Oxygen Flame作者:Hiroshi Naraoka、Michiaki Takasaki、Shinya Nomoto、Kaoru HaradaDOI:10.1246/bcsj.60.414日期:1987.15,6-Dihydro-2,4(1H,3H)-pyrimidinediones underwent dehydrogenation at the 5- and 6-position producing 2,4(1H,3H)-pyrimidinediones in aqueous solution induced by argon arc plasma or flames. From consideration based on the kinetic study of this reaction, it was found that abstraction of a hydrogen atom took place preferentially at the 6-position of the dihydropyrimidinediones.
-
Compositions and method for activating oxygen utilizing N-acylated申请人:Henkel Kommanditgesellschaft auf Aktien公开号:US04110242A1公开(公告)日:1978-08-29The method of activating aqueous solutions of percompounds utilizing N-acylated uracils and benzouracils as activators; solid activated compositions comprising solid percompounds and N-acylated uracils and benzouracils as activators; and novel diacylated uracils and benzouracils.
表征谱图
-
氢谱1HNMR
-
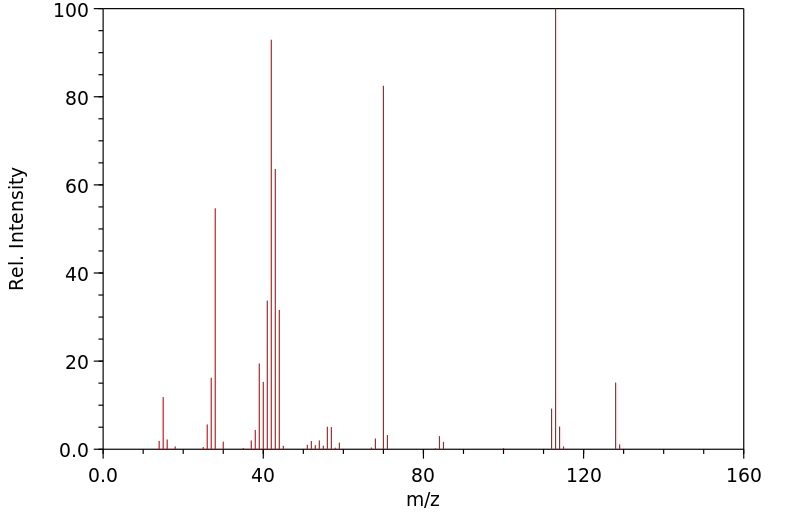
质谱MS
-
碳谱13CNMR
-
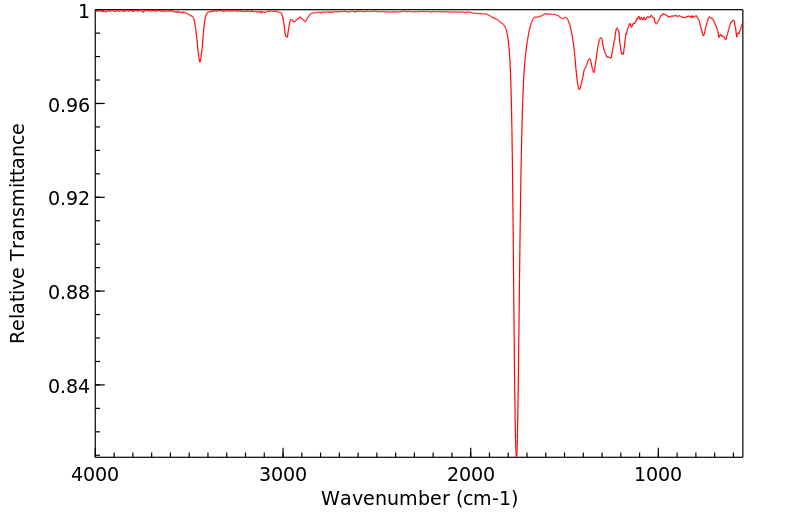
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3