5-羟基戊酰胺 | 29686-12-2
中文名称
5-羟基戊酰胺
中文别名
——
英文名称
5-pentanolide
英文别名
5-hydroxy-valeric acid amide;5-Hydroxy-valeriansaeure-amid;5-Hydroxy-valeramid;5-hydroxy-pentanamide;4-hydroxybutylformamide;5-hydroxy-valeramide;Pentanamide, 5-hydroxy-;5-hydroxypentanamide
CAS
29686-12-2
化学式
C5H11NO2
mdl
——
分子量
117.148
InChiKey
UYOWQFWKDDJSLV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:8
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:63.3
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2924199090
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340,P305+P351+P338,P312,P362,P403+P233,P501
-
危险性描述:H315,H319,H335
SDS
反应信息
-
作为反应物:描述:参考文献:名称:A Mild and Facile Synthesis of Cyclic Imides using Pyridinium Chlorochromate摘要:本文介绍了一种温和、简便的环状亚胺合成方法。这些化合物被广泛用于合成新型医用、聚合、光子和电子材料。与传统合成方法相比,该方法具有条件温和、操作简单和成本低廉等优点。DOI:10.3184/174751911x13202290249578
-
作为产物:参考文献:名称:A Mild and Facile Synthesis of Cyclic Imides using Pyridinium Chlorochromate摘要:本文介绍了一种温和、简便的环状亚胺合成方法。这些化合物被广泛用于合成新型医用、聚合、光子和电子材料。与传统合成方法相比,该方法具有条件温和、操作简单和成本低廉等优点。DOI:10.3184/174751911x13202290249578
文献信息
-
A mild hydration of nitriles into amides作者:P. Breuilles、R. Leclerc、D. UguenDOI:10.1016/s0040-4039(00)76229-0日期:1994.2Stirring mixtures of β-hydroxynitriles with manganese dioxide, deposited onto silica gel for a few days at room temperature resulted in the formation of the corresponding amides in fair to good yields. The unprecedented conversion of 3-hydroxyglutarodinitrile into the corresponding monoamide has been performed by this methodology.
-
[EN] THIAZOLE COMPOUNDS AS INTEGRIN RECEPTOR ANTAGONISTS DERIVATIVES<br/>[FR] COMPOSES DE THIAZOLE EN TANT QUE DERIVES D'ANTAGONISTES DES RECEPTEURS DE L'INTEGRINE申请人:PHARMACIA CORP公开号:WO2004058760A1公开(公告)日:2004-07-15The present invention relates to pharmaceutical compositions comprising compounds of the Formula (I), and methods of selectively inhibiting or antagonizing the ανβ3 and/or the ανβ5 integrin without significantly inhibiting the ανβ6 integrin.本发明涉及包含式(I)化合物的药物组合物,以及选择性抑制或拮抗ανβ3和/或ανβ5整合素而不显著抑制ανβ6整合素的方法。
-
Pyridine-3-carboxamide derivatives as CB1 inverse agonists申请人:Hebeisen Paul公开号:US20060229326A1公开(公告)日:2006-10-12The present invention relates to compounds of the formula wherein X and R 1 to R 8 are as defined in the description and claims, and pharmaceutically acceptable salts thereof. The compounds are useful for the treatment and/or prophylaxis of diseases which are associated with the modulation of CB1 receptors, such as obesity.本发明涉及以下式子的化合物:其中X和R1至R8如描述和权利要求中所定义,并且其药学上可接受的盐。该化合物可用于治疗和/或预防与CB1受体调节有关的疾病,如肥胖症。
-
Protein Lysine Methyltransferase G9a Inhibitors: Design, Synthesis, and Structure Activity Relationships of 2,4-Diamino-7-aminoalkoxy-quinazolines.作者:Feng Liu、Xin Chen、Abdellah Allali-Hassani、Amy M. Quinn、Tim J. Wigle、Gregory A. Wasney、Aiping Dong、Guillermo Senisterra、Irene Chau、Alena Siarheyeva、Jacqueline L. Norris、Dmitri B. Kireev、Ajit Jadhav、J. Martin Herold、William P. Janzen、Cheryl H. Arrowsmith、Stephen V. Frye、Peter J. Brown、Anton Simeonov、Masoud Vedadi、Jian JinDOI:10.1021/jm100478y日期:2010.8.12Protein lysine methyltransferase G9a, which catalyzes methylation of lysine 9 of histone H3 (H3K9) and lysine 373 (K373) of p53, is overexpressed in human cancers. Genetic knockdown of G9a inhibits cancer cell growth, and the dimethylation of p53 K373 results in the inactivation of p53. Initial SAR exploration of the 2,4-diamino-6,7-dimethoxyquinazoline template represented by 3a (BIX01294), a selective small molecule inhibitor of G9a and GLP, led to the discovery of 10 (UNC0224) as a potent G9a inhibitor with excellent selectivity. A high resolution X-ray crystal structure of the G9a-10 complex, the first cocrystal structure of G9a with a small molecule inhibitor, was obtained. On the basis of the structural insights revealed by this cocrystal structure, optimization of the 7-dimethylaminopropoxy side chain of 10 resulted in the discovery of 29 (UNC0321) (Morrison K(i) = 63 pM), which is the first G9a inhibitor with picomolar potency and the most potent G9a inhibitor to date.
-
Synthesis of 2,5-thiazole butanoic acids as potent and selective αvβ3 integrin receptor antagonists with improved oral pharmacokinetic properties作者:John A. Wendt、Hongwei Wu、Heather G. Stenmark、Mark L. Boys、Victoria L. Downs、Thomas D. Penning、Barbara B. Chen、Yaping Wang、Tiffany Duffin、Mary Beth Finn、Jeffery L. Keene、V. Wayne Engleman、Sandra K. Freeman、Melanie L. Hanneke、Kristen E. Shannon、Maureen A. Nickols、Christina N. Steininger、Marissa Westlin、Jon A. Klover、William Westlin、G. Allen Nickols、Mark A. RussellDOI:10.1016/j.bmcl.2005.11.017日期:2006.2We describe a series of 2,5 thiazole containing compounds, which are potent antagonists of the integrin alpha(V)beta(3) and show selectivity relative to the other integrins, such as alpha(IIB)beta(3) and alpha(V)beta(6). These analogs were demonstrated to have high bioavailability relative to other relative heterocyclic analogs. (c) 2005 Elsevier Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
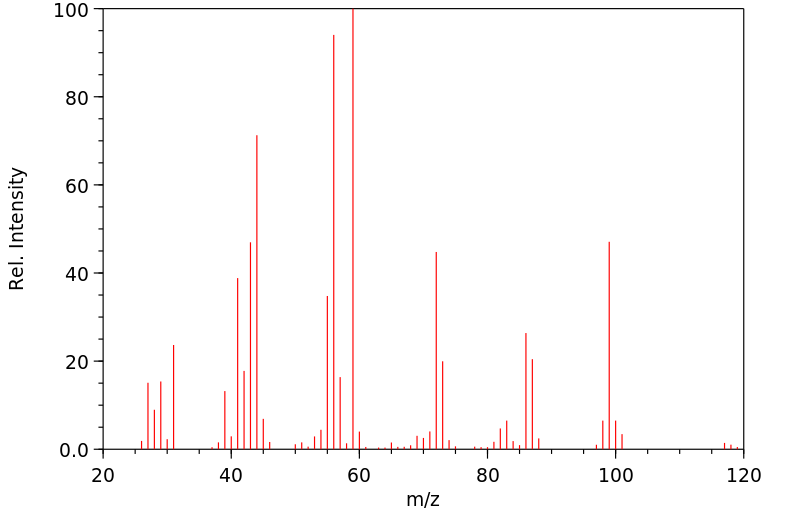
质谱MS
-
碳谱13CNMR
-
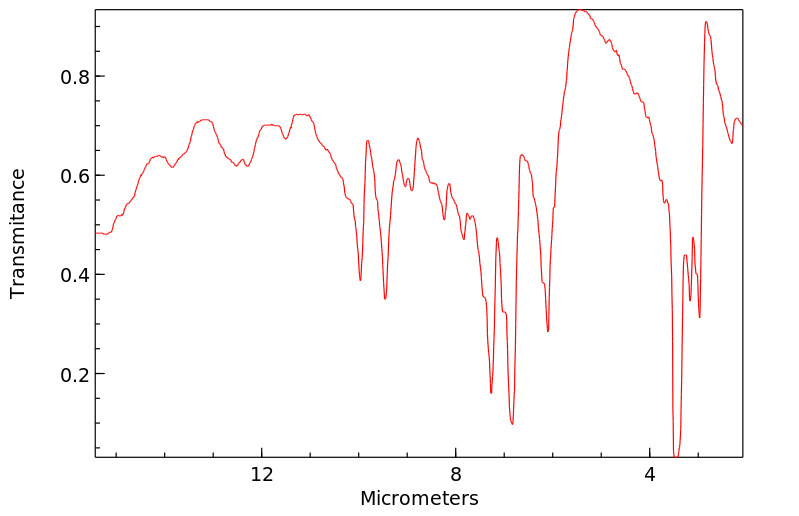
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯