epipregnanolone acetate | 2394-44-7
分子结构分类
中文名称
——
中文别名
——
英文名称
epipregnanolone acetate
英文别名
3β-acetoxy-5β-pregnan-20-one;5beta-Pregnan-20-one, 3beta-hydroxy-, acetate;[(3S,5R,8R,9S,10S,13S,14S,17S)-17-acetyl-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate
CAS
2394-44-7
化学式
C23H36O3
mdl
——
分子量
360.537
InChiKey
GFHOQCXDABGYAL-AOUZBUNXSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.4
-
重原子数:26
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.91
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (3alpha,5beta)-3-(乙酰氧基)孕甾烷-20-酮 3α-acetoxy-5β-pregnan-20-one 1491-77-6 C23H36O3 360.537 —— acetyllithocholic acid 4057-84-5 C26H42O4 418.617 3Beta-羟基-5-beta-妊娠-20-酮 3β-hydroxy-5β-pregnan-20-one 128-21-2 C21H34O2 318.5 21-羟基孕烷醇酮 tetrahydrodeoxycorticosterone 567-03-3 C21H34O3 334.499 —— 3α-acetoxy-24-nor-5β-chol-22-ene 50630-72-3 C25H40O2 372.591 (3R,5R,8R,9S,10S,13S,14S,17S)-3-羟基-10,13-二甲基-2,3,4,5,6,7,8,9,11,12,14,15,16,17-十四氢-1H-环戊二烯并[a]菲-17-羧酸 3α-hydroxy-5β-androstan-17β-carboxylic acid 38775-99-4 C20H32O3 320.472 5beta-孕甾烷-3,20-二酮 pregnanedione 128-23-4 C21H32O2 316.484 孕烯醇酮醋酸酯 Pregnenolone acetate 1778-02-5 C23H34O3 358.521 3b-羟基-5b-孕甾-16-烯-20-酮乙酸酯 3β-acetoxy-5β-pregn-16-en-20-one 2601-07-2 C23H34O3 358.521 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3Beta-羟基-5-beta-妊娠-20-酮 3β-hydroxy-5β-pregnan-20-one 128-21-2 C21H34O2 318.5 —— 3β-acetoxy-17-hydroxy-5β-pregnan-20-one 5456-44-0 C23H36O4 376.536 —— methyl 3β-hydroxy-5β-androstane-17β-carboxylate 10002-84-3 C21H34O3 334.499 3beta,17alpha-二羟基-5beta-孕甾烷-20-酮 5β-pregnan-3β,17α-diol-20-one 570-53-6 C21H34O3 334.499
反应信息
-
作为反应物:参考文献:名称:Partial Synthesis of Compounds Related to Adrenal Cortical Hormones. XIV. Preparation of the Dihydroxyacetone Side Chain; 17α-Hydroxyprogesterone and “Substances L and P”1摘要:DOI:10.1021/ja01145a064
-
作为产物:描述:(3alpha,5beta)-3-(乙酰氧基)孕甾烷-20-酮 在 吡啶 、 sodium hydroxide 、 sodium periodate 、 草酰氯 、 硫酸 作用下, 以 甲醇 、 乙醇 、 水 、 苯 为溶剂, 反应 150.0h, 生成 epipregnanolone acetate参考文献:名称:Preparation of 3β-Acetoxy-5β-pregnan-20-one from 3α-Acetoxy-5β-cholan-24-oic Acid Aimed at the Isotopic Labelling of the Pregnane Side Chain摘要:
摘要 3α-乙酰氧-5β-胆烷-24-酸通过一系列反应转化为3β-乙酰氧-5β-孕烷-20-酮,这些反应涉及在C-3处的构型反转和胆汁酸侧链的降解。该过程将允许在最终化合物的C-21处引入标记。
DOI:10.1515/znb-1984-1125
文献信息
-
Studies on the synthesis of cardiotonic steroids. I. Efficient synthesis of cardenolides.作者:EIICHI YOSHII、TORU KOIZUMI、HIROKAZU IKESHIMA、KYUICHI OZAKI、ICHIHIRO HAYASHIDOI:10.1248/cpb.23.2496日期:——Practical synthetic methods of natural cardenolides are illustrated in the syntheses of xysmalogenin and digitoxigenin. The new routes employ pregn-14-en-20-one as key intermediate which was prepared by partial reduction of 14, 16-dien-20-one with triphenylstannane or triethylsilane. A general and efficient method was devised for obtaining cardenolides consisting of (1) 21-methylsulfenylation of pregnen-20-one, (2) the reaction of the resulting 21-methylthio derivative with bromoacetate and zinc, (3) alumina chromatography of epoxy ester obtained from the S-methylated Reformatsky product.
-
Pyridinium dichromate in organic synthesis: a convenient oxidation of α-ynol-iodine complexes to α,β-unsaturated-α-iodo-aldehydes作者:R. Antonioletti、M. D'Auria、G. Piancatelli、A. ScettriDOI:10.1016/s0040-4039(01)82860-4日期:1981.1Under mild and simple conditions PDC oxidizes α-ynol-l2 complexes to the title carbonyl compounds.在温和简单的条件下,PDC将α-ynol- 12络合物氧化为标题羰基化合物。
-
Attanasi, Orazio; Filippone, Paolino; Serra-Zanetti, Franco, Synthetic Communications, 1982, vol. 12, # 14, p. 1155 - 1162作者:Attanasi, Orazio、Filippone, Paolino、Serra-Zanetti, FrancoDOI:——日期:——
-
Steroidal Sapogenins. XXVII. Preparation and Properties of 20-Isosapogenins<sup>2</sup>作者:Monroe E. Wall、Henry A. WalensDOI:10.1021/ja01626a058日期:1955.11
-
Sterols. LXXXVII. Cholesterol and Sitosterol Derivatives作者:Russell E. Marker、Ewald RohrmannDOI:10.1021/ja01860a016日期:1940.3
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
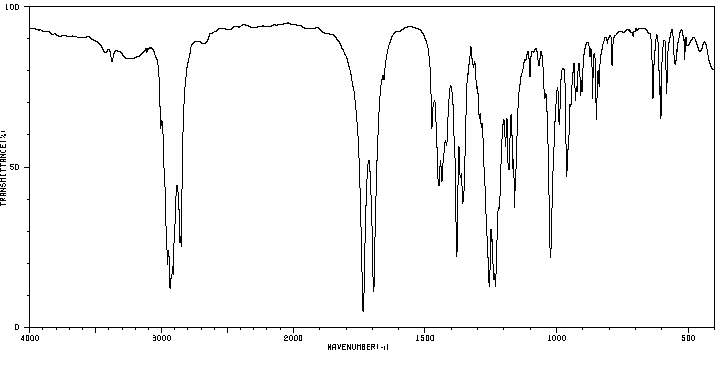
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B