4-hydroxy-8-methoxynaphthalene-1,2-dione | 13261-50-2
中文名称
——
中文别名
——
英文名称
4-hydroxy-8-methoxynaphthalene-1,2-dione
英文别名
2-hydroxy-8-methoxy-[1,4]naphthoquinone;2-hydroxy-8-methoxy-1,4-naphthoquinone;3-Hydroxy-5-methoxy-1,4-naphthoquinone;3-Hydroxy-5-methoxy-naphthochinon-(1,4);2-Hydroxy-8-methoxy-1,4-naphthochinon;Hydroxyjuglon-methylether
CAS
13261-50-2
化学式
C11H8O4
mdl
——
分子量
204.182
InChiKey
FYKICSYZKOGCIS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:211-214 °C (decomp)(Solv: ethanol (64-17-5))
-
沸点:414.3±45.0 °C(Predicted)
-
密度:1.442±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.52
-
重原子数:15.0
-
可旋转键数:1.0
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:63.6
-
氢给体数:1.0
-
氢受体数:4.0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2,8-dimethoxynaphthalene-1,4-dione 52280-74-7 C12H10O4 218.209 8-甲氧基-1,4-萘醌 5-methoxynaphthoquinone 4923-61-9 C11H8O3 188.183 2-溴-8-甲氧基萘-1,4-二酮 3-bromo-5-methoxy-1,4-naphthoquinone 69833-10-9 C11H7BrO3 267.079 —— 2-amino-8-methoxynaphthalene-1,4-dione 56568-58-2 C11H9NO3 203.197 5-羟基对萘醌 5-hydroxynaphtho-1,4-quinone 481-39-0 C10H6O3 174.156 8-甲氧基-Alpha-四氢萘酮 8-methoxy-1-tetralone 13185-18-7 C11H12O2 176.215 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4,8-dihydroxynaphthalene-1,2-dione 4923-58-4 C10H6O4 190.155 —— 2,8-diacetoxy-[1,4]naphthoquinone 61276-36-6 C14H10O6 274.23 —— 9-methoxy-2,2-dimethyl-2H-naphtho<2,3-b>pyran-5,10-dione 58785-62-9 C16H14O4 270.285 —— 9-methoxy-2-(1'-propyl)-3,4-dehydronaphtho[2,3-b]pyran-5,10-dione 700816-44-0 C17H16O4 284.312 —— 2-isopropenyl-8-methoxy-2,3-dihydronaphtho[2,3-b]furan-4,9-dione —— C16H14O4 270.285 —— 9-methoxy-2-[(2-methyl-1,3-dioxolan-2-yl)methyl]-2H-benzo[g]chromene-5,10-dione 700816-47-3 C19H18O6 342.348
反应信息
-
作为反应物:参考文献:名称:Giles,R.G.F.; Roos,G.H.P., Journal of the Chemical Society. Perkin transactions I, 1976, p. 1632 - 1635摘要:DOI:
-
作为产物:描述:甲氧苯醌 在 sodium hydroxide 、 ammonium cerium(IV) nitrate 作用下, 以 水 、 乙腈 、 苯 为溶剂, 反应 2.5h, 生成 4-hydroxy-8-methoxynaphthalene-1,2-dione参考文献:名称:碱性条件下甲氧基-2-羟基-1,4-萘醌的合成及一种异构体与醛的反应摘要:摘要 研究了合成甲氧基-2-羟基-1,4-萘醌的两种方案,以评估它们在胺碱性条件下对醛的行为。醌和脂肪族醛的性质都有助于这种缩合的可行性以及进一步的转化。DOI:10.1081/scc-120030312
文献信息
-
One-Step Synthesis of Naphthofurandione, Benzofurandione, and Phenalenofuranone Derivatives by the CAN-Mediated Cycloaddition作者:Kazuhiro Kobayashi、Tomokazu Uneda、Koujirou Tanaka、Masako Mori、Hideo Tanaka、Osamu Morikawa、Hisatoshi KonishiDOI:10.1246/bcsj.71.1691日期:1998.7The 3+2-type cycloaddition reaction of 2-hydroxy-1,4-naphthoquinones with various alkenes or phenylacetylene was mediated by ammonium cerium(IV) nitrate (CAN) to give the corresponding naphtho[2,3-b]furan-4,9-dione and naphtho[1,2-b]furan-4,5-dione derivatives. The reaction of 2-hydroxy-1,4-benzoquinones with alkenes in the presence of CAN similarly proceeded to give benzofuran-4,7-dione and benzofuran-4,5-dione derivatives. 3-Hydroxy-1H-phenalen-1-one also underwent the CAN-mediated cycloaddition with alkenes or phenylacetylene to give the corresponding 7H-phenaleno[1,2-b]furan-7-one derivatives.
-
Synthesis, Characterization and Cytotoxic Investigations of Novel C3-Dihydrofuran Substituted 1H-benzo[g]chromene-2,5,10-triones besides Antimicrobial study作者:Venkata Swamy Tangeti、D. Vasundhara、M. Naresh Kumar、Himabindu Mylapalli、Kaja Srinivas Pavan KumarDOI:10.14233/ajchem.2017.20209日期:——A series of novel C3-dihydrofuran substituted 1H-benzo[g]chromene-2,5,10-triones were synthesized and characterized by spectral analysis. The target molecules were screened for their antimicrobial and anticancer activities and structure and activity relationship (SAR) was investigated. Structure and activity relationship studies revealed that the compounds 6p, 6q, 6s, 6t were found to be more active in antimicrobial screening. Antiproliferative properties were evaluated against human cancer cell lines, namely, laryngeal carcinoma (Hep2), lung adenocarcinoma (A549) and cervical cancer (HeLa). The best among them, C3-dihydrofuran substituted 1H-benzo[g]chromene-2,5,10-trione with methoxy group substitution at ring A and B were selected for further structure activity relationship. Among the derivatives, (2S,3S)-propyl-4-acetyl-5-(7,9-dimethoxy-2,5,10-trioxo-5,10-dihydro-2H-benzo[g]chromen-3-yl)-3-(3,5-dimethoxyphenyl)-2,3-dihydrofuran-2-carboxylate (6t) showed most potent cytotoxic activity against all the three cancer cell lines. Toxicity studies revealed that dihydrofuran substituted 1H-benzo[g] chromene-2,5,10-triones (6a-t) are specifically target the cancer cell lines.合成了一系列新型C3-二氢呋喃取代的1H-苯并[g]色烯-2,5,10-三酮,并通过光谱分析对其进行了表征。针对这些靶分子进行了抗微生物和抗癌活性的筛选,并研究了其结构与活性关系(SAR)。结构与活性关系研究显示,化合物6p、6q、6s、6t在抗微生物筛选中表现更为活跃。抗增殖特性则针对人类癌细胞系进行评估,包括喉癌(Hep2)、肺腺癌(A549)和宫颈癌(HeLa)。在这些化合物中,C3-二氢呋喃取代的1H-苯并[g]色烯-2,5,10-三酮A和B环上有甲氧基取代的化合物被选为进一步的结构活性关系研究。在这些衍生物中,(2S,3S)-丙基-4-乙酰基-5-(7,9-二甲氧基-2,5,10-三氧-5,10-二氢-2H-苯并[g]色烯-3-基)-3-(3,5-二甲氧基苯基)-2,3-二氢呋喃-2-羧酸酯(6t)对所有三种癌细胞系表现出最强的细胞毒活性。毒性研究表明,二氢呋喃取代的1H-苯并[g]色烯-2,5,10-三酮(6a-t)特异性靶向癌细胞系。
-
Synthesis and Cytotoxicity on Human Leukemia Cells of Furonaphthoquinones Isolated from <i>Tabebuia</i> Plants作者:Ryuta Inagaki、Masayuki Ninomiya、Kaori Tanaka、Kunitomo Watanabe、Mamoru KoketsuDOI:10.1248/cpb.c13-00011日期:——Furonaphthoquinones are promising skeletons for anticancer drug molecules. In particular, methoxylated furonaphthoquinones are characteristic constituents of Tabebuia plants. In this research, we synthesized the furonaphthoquinones by effective one-pot cascade reactions of 3-phenyliodonio-1,2,4-trioxo-1,2,3,4-tetrahydronaphthalenides with 3-butyn-2-ol in the presence of palladium and cuprous catalysts via Sonogashira coupling and intramolecular cyclization. Furthermore, we demonstrated that the synthetic furonaphthoquinones showed moderate cytotoxicity against human leukemia U937 and HL-60 cells. Our work highlights the importance of furonaphthoquinones as antileukemic agents.
-
One-step preparation of some 2-isopropenyl-2,3-dihydronaphtho-[2,3-<i>b</i>]furan-4,9-diones作者:Seiji Yamaguchi、Toshiharu Katsuki、Hajime Yokoyama、Yoshiro HiraiDOI:10.1002/jhet.5570380235日期:2001.3Some 2-isopropenyl-2,3-dihydronaphtho[2,3-b]furan-4,9-diones la-f,b',f were prepared by one-step cyclizations of 2-hydroxy-1,4-naphthoquinones 2a-f with 1,4-dibromo-2-methyl-2-butene (3).
-
Photochemical Reaction of 1,2-Naphthoquinone toward Enantioselective Synthesis of γ-Rubromycin: Viability Dependence on Chromophore作者:Yoshio Ando、Ken Ohmori、Daichi Ogawa、Fumihiro Wakita、Keisuke SuzukiDOI:10.1055/a-2113-0212日期:2024.3at the enantioselective total synthesis of γ-rubromycin, we reported the photochemical reaction of 1,2-naphthoquinone as a promising solution to otherwise-difficult enantiocontrol of the single spiroacetal stereogenic center. The present study examined the applicability of this approach to more functionalized substrates, which revealed viability dependence on the chromophore structure differing in the
表征谱图
-
氢谱1HNMR
-
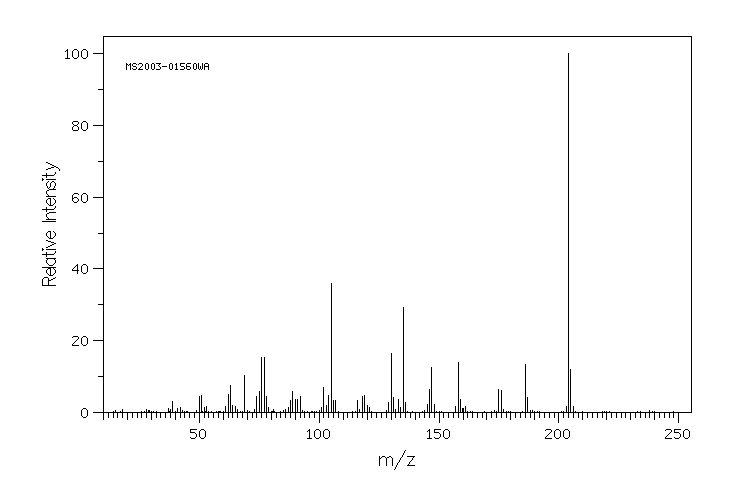
质谱MS
-
碳谱13CNMR
-
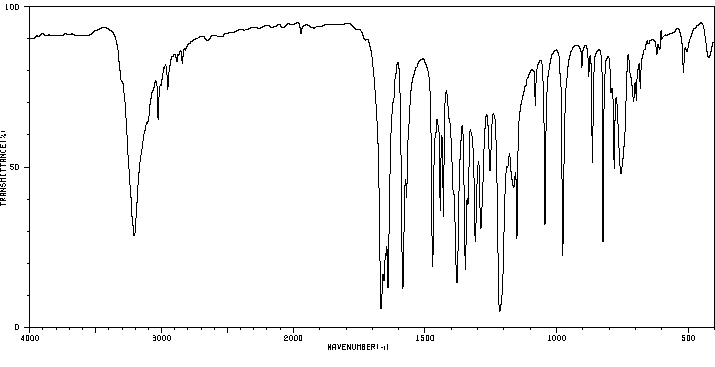
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮