9,10-二氢-9,10-乙桥蒽 | 5675-64-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:16
-
可旋转键数:0
-
环数:5.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 11-chloroethanoanthracene 6476-45-5 C16H13Cl 240.732 9,10-二氢-9,10-乙烯桥蒽 9,10-dihydro-9,10-etheno-anthracene 2734-13-6 C16H12 204.271 —— O-<(5,7-dibenzobicyclo<2.2.2>octa-5,7-dien-2-yl)-methyl> S-methyl xanthate 79134-80-8 C18H16OS2 312.456 —— (10H-9,10-ethano-anthracen-9-yl)-hydrazine 119247-28-8 C16H16N2 236.316 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-amino-9,10-ethano-9,10-dihydroanthracene 107125-77-9 C16H15N 221.302 —— 9,10-ethano-9,10-dihydro-2,3-diiodoanthracene 107125-83-7 C16H12I2 458.08 —— 9,10-dihydro-9,10-ethano-anthracen-11-one 6372-63-0 C16H12O 220.271 —— 11-chloroethanoanthracene 6476-45-5 C16H13Cl 240.732 —— 2-acetyl-9,10-dihydro-9,10-ethanoanthracene 80735-59-7 C18H16O 248.324 —— 2-acetylamino-9,10-ethano-9,10-dihydroanthracene 107125-78-0 C18H17NO 263.339 —— 2-neo-pentylamino-9,10-ethano-9,10-dihydroanthracene 107125-90-6 C21H25N 291.436 —— 2-amino-9,10-ethano-9,10-dihydro-3-iodoanthracene 107125-82-6 C16H14IN 347.198 —— 2-(2,2-dimethylpropanoylamino)-9,10-ethano-9,10-dihydroanthracene 107125-86-0 C21H23NO 305.42 —— 2-amino-9,10-ethano-9,10-dihydro-3-nitroanthracene 107125-81-5 C16H14N2O2 266.299 —— 2-acetylamino-9,10-ethano-9,10-dihydro-3-iodoanthracene 107125-84-8 C18H16INO 389.236 - 1
- 2
反应信息
-
作为反应物:描述:9,10-二氢-9,10-乙桥蒽 在 吡啶 、 palladium on activated charcoal 、 碘 、 硝酸 、 乙酸酐 、 potassium carbonate 、 一水合肼 、 三乙胺 、 palladium dichloride 作用下, 以 乙醚 、 硝基甲烷 、 乙醇 、 二氯甲烷 、 水 为溶剂, 100.0 ℃ 、413.69 kPa 条件下, 反应 89.5h, 生成 methyl 3-acetylamino-9,10-ethano-9,10-dihydroanthracene-2-carboxylate参考文献:名称:取代的乙基蒽的制备和热解-2,3-二取代的蒽的一般合成摘要:已经对9,10-乙烷-9,10-二氢蒽进行了区域选择性官能化,得到了一系列2-取代和2,3-二取代的衍生物。这些化合物的快速真空热解提供了2,3-二取代的蒽的第一个一般合成。观察到的区域选择性符合与芳环稠合的应变环的电子影响的Finnegan-Streitwieser模型。DOI:10.1016/s0040-4020(01)87549-9
-
作为产物:描述:蒽 在 Lindlar's catalyst 、 乙醇 、 zinc stearate 、 甲苯 、 乙炔 作用下, 180.0~200.0 ℃ 、2.45 MPa 条件下, 生成 9,10-二氢-9,10-乙桥蒽参考文献:名称:DE932126摘要:公开号:
文献信息
-
Reductive decarboxylation of N-(acyloxy)phthalimides via redox-initiated radical chain mechanism作者:Keiji Okada、Katsura Okubo、Naoto Morita、Masaji OdaDOI:10.1016/s0040-4039(00)60192-2日期:1992.11Highly efficient reductive decarboxylation of N-(acyloxy)phthalimides which are readily prepared from carboxylic acids was achieved by visible light irradiation using Ru(bpy)3Cl2 as a sensitizer in the presence of BNAH and t-BuSH via radical chain mechanism.
-
Substitution of Bridgehead Halogens by a Free-Radical Electron-Transfer Mechanism作者:MC Harsanyi、PA Lay、RK Norris、PK WittingDOI:10.1071/ch9960581日期:——
The reactions of 1-bromo-7-nitro- and 1-bromo-6-nitro-1,4-methanonaphthalene (2) and (3), and 9-bromo-2-nitro, 10-bromo-2-nitro-, 9,10-dibromo-2-nitro- and 9,10-diiodo-2-nitro-9,10-ethano-9,10-dihydroanthracene (4)-(7). respectively, with the sodium salt (1) of p-toluenethiol gave substitution products that were shown to be formed by an SRN1 or a related radical chain mechanism. In the relatively slow substitution reactions of the salt (1) with compounds (2)-(5). That contain bromine at bridgehead positions that are either meta- or para-benzylic to an aromatic nitro group, the rates of substitution in the isomers where the leaving group was meta- benzylic to the aromatic nitro group were slightly greater than those for the corresponding para-benzylic isomer. In compounds (6)and (7) the halogens are at bridgehead positions that are either meta- or para-benzylic relative to an aromatic nitro group within the same molecule. In the case of the reaction of the dibromide (6) with the thiolate (1), substitution was slow and occurred more rapidly at the benzylic -bridgehead position meta to the nitro group than at the corresponding para-benzylic position. In contast , the reaction of the diiodide (7) with the thiolate (1) gave substitution products which formed more rapidly than in the corresponding reaction of the dibromide (6) and the regioselectivity was reversed, with substitution occurring more readily at the bridgehead position para-benzylic to the nitro group than at the corresponding meta- benzylic position. The ratio of meta to para substitution products, determined for the reactions of compounds (2)-(6) with the salt (1), were in the range 1.15-2.5:1, while the reaction of (7) with the same nucleophile afforded a meta-to-para ratio of 1:2:3. These ratios contrast not only with each other, but also with the differences in reactivities determined for other nitrobenzylic systems, which are known to undergo SRN1 substitution reactions with the same nucleophile. The differences in first, the regioselectivity of substitution between the bridgehead systems, and secondly, the differences in the observed rates of regioselective substitution are compared with other simple nitrobenzylic halides. These differences are rationalized in terms of the effect of fixing the C-X bond at a bridgehead position to be orthogonal with the plane of the nitroaromatic group; this results in a reduction of the rate constants of intramolecular electron transfer, with significant consequences on the detailed overall mechanism for these reactions.
1-溴-7-硝基-和1-溴-6-硝基-1,4-甲基萘(2)和(3),以及9-溴-2-硝基,10-溴-2-硝基,9,10-二溴-2-硝基和9,10-二碘-2-硝基-9,10-乙烯基-9,10-二氢蒽(4)-(7)的反应。分别与对甲苯硫醇(1)的钠盐反应,形成了被证明是通过SRN1或相关的自由基链机制形成的取代产物。在盐(1)与含有溴在桥头位置的化合物(2)-(5)之间相对缓慢的取代反应中。其中溴位于对芳基硝基的间位或对位,离去基团为对芳基硝基的异构体的取代速率略高于相应的对位取代体。在化合物(6)和(7)中,卤素位于相同分子中相对于芳基硝基的桥头位置的间位或对位。在二溴化物(6)与硫醇酸盐(1)的反应中,取代较慢,并且在间位于硝基的芳基桥头位置比相应的对位芳基位置更快地发生。相反,二碘化物(7)与硫醇酸盐(1)的反应产生的取代产物比二溴化物(6)的相应反应更快形成,而且取代选择性被颠倒,取代更容易发生在对硝基的芳基桥头位置,而不是相应的间位芳基位置。对于化合物(2)-(6)与盐(1)的反应确定的间位和对位取代产物比率在1.15-2.5:1的范围内,而(7)与相同亲核试剂的反应得到了1:2:3的间位与对位比率。这些比率不仅彼此之间有所不同,而且与已知与相同亲核试剂发生SRN1取代反应的其他硝基芳基系统的反应性差异也不同。比较了桥头系统之间的取代选择性的差异,以及观察到的取代选择性取代速率的差异与其他简单硝基芳基卤化物。这些差异是根据将C-X键固定在桥头位置使其与硝基芳基平面正交的效应来解释的;这导致分子内电子转移速率常数的降低,对这些反应的详细整体机制产生重要影响。 -
Conformational analysis of 9,10-dihydroanthracenes. Molecular mechanics calculations and carbon-13 NMR作者:Peter W. Rabideau、Jennifer L. Mooney、Kenny B. LipkowitzDOI:10.1021/ja00286a002日期:1986.12The conformational analyses of 9, 10-dihydroanthracene and several of its methylated and ethylated derivatives are studied by empirical force field calculations (MM2 and MMPI). The computational results are considered in light of previous and current carbon NMR data. Model compounds are examined which involve fixed, planar, and boat-shaped conformations about the central ring, and these /sup 13/C NMR
-
Reductive desulfonylation of phenyl sulfones by samarium(II) iodide-hexamethylphosphoric triamide作者:H. Künzer、M. Stahnke、G. Sauer、R. WiechertDOI:10.1016/0040-4039(91)85009-t日期:1991.4Samarium(II) iodide in tetrahydrofuran reductively desulfonylates phenyl sulfones in the presence of hexamethylphosphoric triamide. This transformation is illustrated here for ten substrates, which include secondary alicyclic, β-hydroxy, vicinal bis-, and α,β-unsaturated sulfones.
-
Über die Desaminierung von Brückenkopf-Aminen. 9-Amino-9,10-dihydro-9,10-äthano-anthracen作者:M. Wilhelm、D. Y. CurtinDOI:10.1002/hlca.19570400712日期:——Durch Anlagerung von Äthylen an 9-Amino-anthracen wurde 9-Amino-9,l0-dihydro-9,10-äthano-anthracen (III) hergestellt. Die Desaminierung dieses Brückenkopf-Amins mit Nitrosylchlorid in Diäthyläther bei −70° gab in rasch verlaufender Reaktion 9-Chlor-9,10-dihydro-9,10-äthano-anthracen (VIII) und 9-Äthoxy-9,l0-dihydro-9,10-äthano-anthracen (IX). Für den Zerfall des intermediär entstehenden 9,10-Dihydro-9Durch Anlagerung vonÄthylen是9-氨基蒽的古德9-氨基-9,10-二氢-9,10-安他诺蒽(III)的雌激素。Dsa Desaminierung在Rasch Verlaufender的Diäthylätherbei -70°gab中使Brückenkopf-Aminsmit亚硝酰氯死亡Reaktion9-Chlor-9,10-dihydro-9,10-äthano-蒽(VIII)和9-Äthoxy-9,l0-dihydro- 9,10-蒽蒽(IX)。毛皮巢穴Zerfall DESintermediärentstehenden 9,10-二氢-9,10-äthano-9-蒽基-diazoniumchlorids(XIII)利瑟SICH EINE minimale Geschwindigkeitskonstante冯10 -2秒-1 abschätzen。Dieser
表征谱图
-
氢谱1HNMR
-
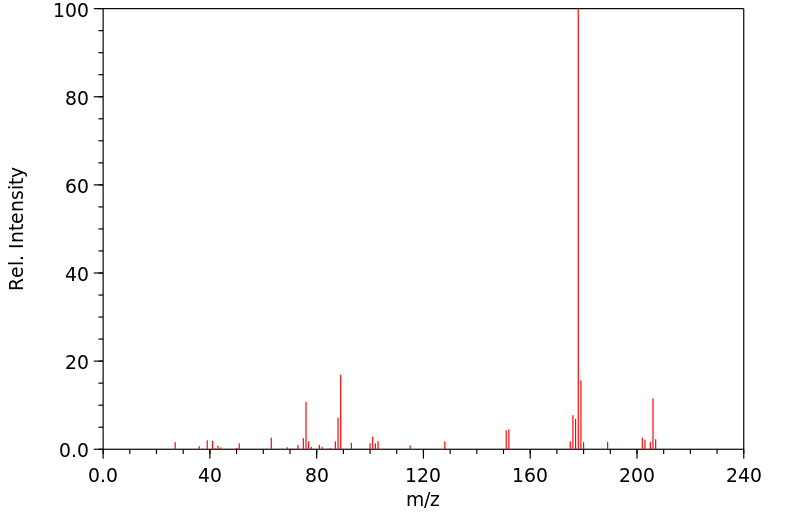
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息