嘧菌环胺 | 121552-61-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:75.9°
-
沸点:406.0±48.0 °C(Predicted)
-
密度:1.21 g/cm3
-
溶解度:氯仿:微溶
-
LogP:4.000
-
物理描述:Solid
-
颜色/状态:Powder with agglomerates at 20 °C
-
气味:Weak odor
-
蒸汽压力:3.68X10-6 mm Hg at 25 °C
-
稳定性/保质期:
常温常压下稳定。
-
分解:DT50 in pH range 4-9 (25 °C) >>1 yr. Photolysis DT50 in water 0.4-13.5 days.
-
解离常数:pKa = 4.44
-
碰撞截面:152.07 Ų [M+H]+ [CCS Type: TW]
-
保留指数:2068;2037;2043;2019.1;2024.4
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:17
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:37.8
-
氢给体数:1
-
氢受体数:3
ADMET
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/38,R43
-
WGK Germany:2
-
危险品运输编号:UN 3077
-
海关编码:2942000000
-
包装等级:III
-
储存条件:密封,在0-6°C下保存
SDS
制备方法与用途
嘧菌环胺是由先正达公司(原Ciba-Geigy AG)开发的杀菌剂,于1994年首次上市。目前该产品已进入专利期外阶段。2007年,先正达在国内进行了临时登记,开启了嘧菌环胺在中国市场的应用。然而,在随后5年内仅取得98%嘧菌环胺原药和62%嘧菌环胺•咯菌腈水分散粒剂的正式登记;直至2014年,才将50%嘧菌环胺水分散粒剂正式注册用于防治葡萄灰霉病。
化学性质嘧菌环胺纯品为粉末状固体,具有轻微气味,熔点75.9℃。其相对密度在20℃时为1.21。蒸气压(25℃):结晶状固体A为5.1×10^-4 Pa,固体B为4.7×10^-4 Pa。分配系数(25℃)Kow值分别为pH 5时3.9、pH 7时4.0和pH 9时4.0。在水中溶解度(25℃,g/L):pH 5时0.020、pH 7时0.013、pH 9时0.015;乙醇中可溶于160 g/L,丙酮中为610 g/L,甲苯中440 g/L,正己烷中26 g/L,正辛醇中140 g/L。离解常数pKa值为4.44。稳定性:在pH 4~9范围内的水溶液(25℃)下DT50>14天;水中光解的DT50为21天(蒸馏水)、13天(pH 7.3)。
作用机理嘧菌环胺是一种蛋氨酸生物合成抑制剂,与三唑类、咪唑类、吗啉类及苯基吡咯类等化合物无交互抗性。
作用对象嘧菌环胺适用于小麦、大麦、葡萄、草莓、果树、蔬菜和观赏植物等多种作物的病害防治。
生物活性Cyprodinil 是一种广谱杀菌剂,可抑制植物病原真菌蛋氨酸的生物合成。在无氨基酸的培养基上,它能抑制多种真菌(B. cinerea、P. herpotrichoides 和 H. oryzae)菌丝体细胞生长,IC50值分别为0.44 µM、4.8 µM和0.03 µM。在没有AR激动剂DHT的情况下,Cyprodinil 可作为雄激素受体(AR)激动剂(EC50=1.91 µM),并能抑制DHT的活性(IC50=15.1 µM)。
安全性嘧菌环胺对作物安全,无药害风险。
合成方法密菌环胺的合成类似于密霉胺。以苯胺、氰氨和环丙酰氯为起始原料,经过一系列反应可得到目标产物。具体步骤如下:
以苯胺、氰氨、环丙酰氯为原料,在特定条件下进行反应,最终制得嘧菌环胺。具体的合成路线图未给出,但可通过文献查阅或咨询相关技术人员了解详细过程。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 嘧菌胺 mepanipyrim 110235-47-7 C14H13N3 223.277 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 嘧菌环胺代谢物CGA304075 Phenol, 4-((4-cyclopropyl-6-methyl-2-pyrimidinyl)amino)- 195157-66-5 C14H15N3O 241.293 4-环丙基-6-甲基-2-嘧啶胺 2-Amino-4-cyclopropyl-6-methyl-pyrimidine 92238-61-4 C8H11N3 149.195
反应信息
-
作为反应物:参考文献:名称:Microbial Transformations of the Fungicide Cyprodinil (CGA-219417)摘要:A collection of 12 microbial cultures, known to contain cytochrome P-450 monooxygenase or other degradative enzymes, was screened for their ability to degrade the Novartis Crop Protection Inc. developmental fungicide cyprodinil (CGA-219417; 4-cyclopropyl-6-methyl-N-phenyl-2-pyrimidinamine). Ten of the 12 cultures produced a monohydroxylated metabolite in yields ranging from 1.2 to 35.6%. The filamentous fungus, Beauveria bassiana ATCC 7159, produced a methoxylated glycoside of the monohydroxylated metabolite with a yield of 80%. Dihydroxylated metabolites and a molecular cleavage product, 4-cyclopropyl-6-methyl-2-pyrimidamine, were also detected in certain cultures. The overall results of the study indicated that cyprodinil was readily metabolized by a variety of microbial species. Metabolites generated by these cultures can potentially be used as analytical reference standards to support animal, plant, and soil metabolism studies.DOI:10.1021/jf970298l
-
作为产物:参考文献:名称:Pesticides摘要:公式为##STR1##的化合物,其中:R.sub.1和R.sub.2彼此独立地是氢、卤素、C.sub.1-C.sub.3烷基、C.sub.1-C.sub.2卤代烷基、C.sub.1-C.sub.3烷氧基或C.sub.1-C.sub.3卤代烷氧基;R.sub.3是氢;C.sub.1-C.sub.4烷基;或由卤素、羟基或氰基取代的C.sub.1-C.sub.4烷基;环丙基;或由甲基和/或卤素单取代至三取代的环丙基;以及R.sub.4是C.sub.3-C.sub.6环烷基或由甲基和/或卤素单取代至三取代的C.sub.3-C.sub.6环烷基,具有有价值的杀菌和杀虫性能。这种新型活性成分可用于植物保护,用于防止植物病原微生物或有害昆虫对栽培植物的侵害,并用于控制这些害虫。公开号:US04931560A1
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] SUBSTITUTED QUINAZOLINES AS FUNGICIDES<br/>[FR] QUINAZOLINES SUBSTITUÉES, UTILISÉES EN TANT QUE FONGICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2010136475A1公开(公告)日:2010-12-02The present invention relates to a compound of formula (I) wherein wherein the substituents have the definitions as defined in claim 1or a salt or a N-oxide thereof, their use and methods for the control and/or prevention of microbial infection, particularly fungal infection, in plants and to processes for the preparation of these compounds.本发明涉及一种具有如下式(I)的化合物,其中取代基具有权利要求1中定义的定义,或其盐或N-氧化物,它们的用途以及用于控制和/或预防植物中微生物感染,特别是真菌感染的方法,以及制备这些化合物的方法。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
表征谱图
-
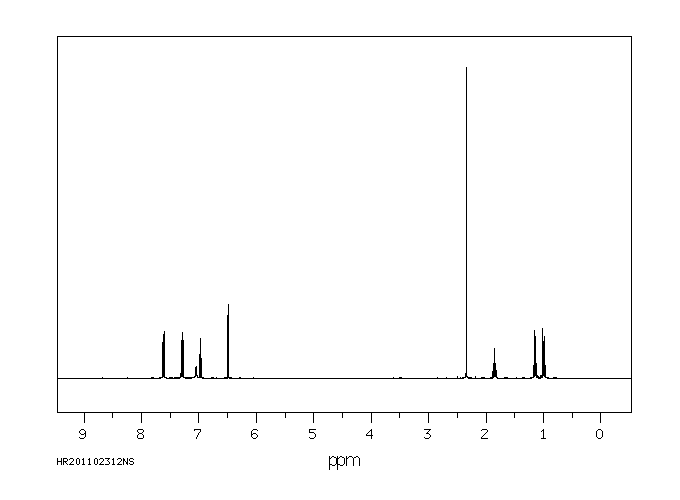
氢谱1HNMR
-
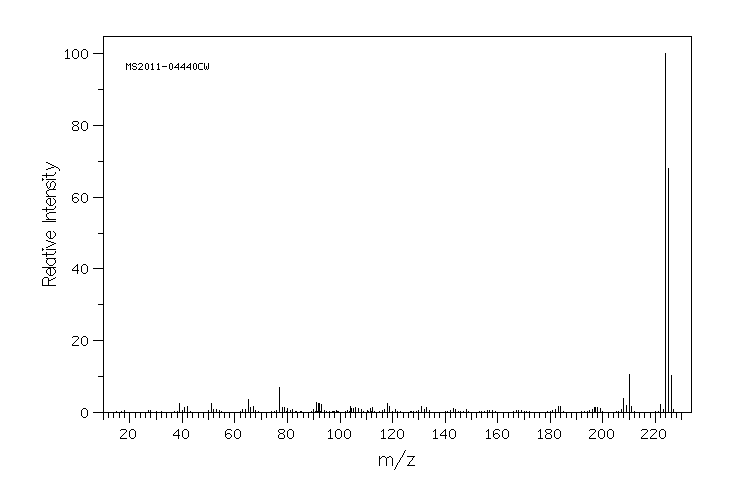
质谱MS
-
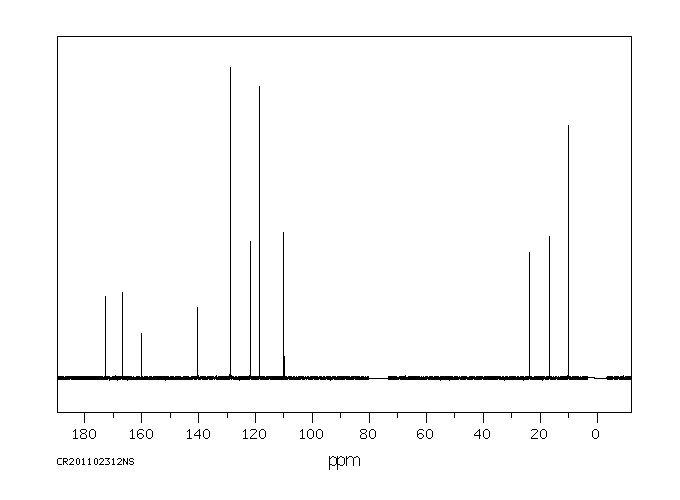
碳谱13CNMR
-
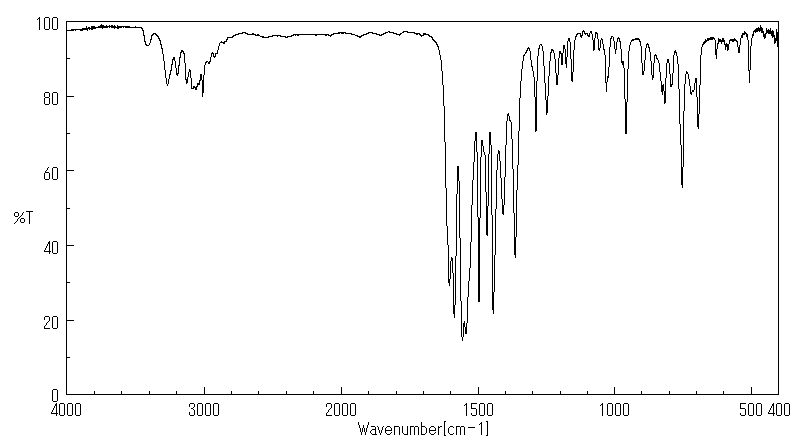
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息