1,3-二溴-1-丙烯 | 32121-06-5
中文名称
1,3-二溴-1-丙烯
中文别名
——
英文名称
(Z)-1,3-dibromopropene
英文别名
1,3-dibromopropene;1,3-Dibromo-1-propene;1c,3-dibromo-propene;1c,3-Dibrom-propen;γ-Brom-allylbromid, cis-Form;cis-1,3-Dibrom-propen;1.3-Dibrom-propen, cis-Form;(Z)-1,3-dibromo-1-propene;(Z)-1,3-dibromoprop-1-ene
CAS
32121-06-5
化学式
C3H4Br2
mdl
——
分子量
199.873
InChiKey
JWQMKMSVOYQICF-UPHRSURJSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:141.92°C (estimate)
-
密度:2.0599
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 顺式-1-溴-1-丙烯 (Z)-1-propenyl bromide 590-13-6 C3H5Br 120.977 1-溴基-1-丙烯 1-bromo-1-propene 590-14-7 C3H5Br 120.977
反应信息
-
作为反应物:描述:参考文献:名称:Fluorinated pentene diamine derivatives摘要:含氟烯基亚胺化合物是体内鸟氨酸脱羧酶的抑制剂,并具有以下公式:##STR1## 其中:R.sub.a 代表氢或R.sub.2,其中R.sub.2 如下定义;R.sub.b 代表氢或,当R.sub.a 是氢时,R.sub.2,其中R.sub.2 如下定义;R.sub.c 代表氢或--COR.sub.3,其中R.sub.3 如下定义;R.sub.1 代表氢或C.sub.1 --C.sub.6 烷基;每个R.sub.2,独立地,代表C.sub.2 -C.sub.5 烷基甲酰基,苯甲酰基,苯基-(C.sub.1 -C.sub.4 烷基)甲酰基,或通过从L-氨基羧酸的一个羧基去除一个羟基团得到的氨基羧酸残基;R.sub.3 代表羟基,或,当R.sub.a 和R.sub.b 都是氢时,C.sub.1 -C.sub.8 烷氧基,--NR.sub.4 R.sub.5,其中R.sub.4 和R.sub.5 如下定义,或通过从L-氨基羧酸的氨基去除一个氢原子得到的氨基羧酸残基;R.sub.4 和R.sub.5,独立地,代表氢或C.sub.1 -C.sub.4 烷基;并且p代表1或2。公开号:US04439619A1
-
作为产物:描述:参考文献:名称:钯催化芳烃羧酸/酯与芳基溴化物的分子内脱羧偶联摘要:给我一个戒指?已经开发出一种有效的方法用于芳烃羧酸/酯与钯催化的芳基溴化物的分子内脱羧偶联(参见方案)。从合成的观点来看,该方法极具吸引力,因为催化剂的负载量低,优化的反应条件温和且底物范围广。DOI:10.1002/chem.201103438
文献信息
-
Total Syntheses of Vicinal Dichloride Monoterpenes Enabled by Aza-Belluš–Claisen Rearrangement作者:Jiangqun Cheng、Yuan-He Li、Jun Huang、Zhen YangDOI:10.1021/acs.orglett.1c03187日期:2021.11.5were achieved via an aza-Belluš–Claisen rearrangement, which involved the reaction of an α-chloro carboxylic acid chloride with halogen-substituted trans-allyl morpholines in the presence of Lewis acids. The developed method was used for the total synthesis of a group of monoterpene natural products bearing vicinal dichloride subunits.
-
A novel synthesis of unsaturated spiro compounds based on reactions of bifunctional organometallic reagents.作者:Perséphone Canonne、Raynald Boulanger、Paul AngersDOI:10.1016/s0040-4039(00)79411-1日期:1991.10Z-allylic dibromides regio- and stereoselectively prepared were reacted with the sodium enolate of ethyl acetoacetate or di(α-ethoxyvinyl)cuprate to yield, after hydrolysis, and decarboxylation in the case where the enolate of ethylacetoacetate was the nucleophile, the corresponding ketones. After reduction and bromination, the products were converted into the appropriate organometallic compounds and
-
Asymmetric Synthesis of a Bicyclo[4.3.0]nonene Derivative Bearing a Quaternary Carbon Stereocenter: Desymmetrization of σ-Symmetrical Diketones through Intramolecular Addition of an Alkenyl Anion作者:Tomoyuki Yoshimura、Yuki Enami、Jun-ichi MatsuoDOI:10.1055/s-0040-1706421日期:2020.12strategy involving an intramolecular addition. The intramolecular nucleophilic addition of a highly reactive carbanion generated from an alkenyl iodide in the presence of a chiral ligand occurs with discrimination of two keto carbonyl groups to give the corresponding bicyclic compound in 81% yield and 39% ee. Asymmetric synthesis via an intramolecular desymmetrization strategy using a chiral ligand–carbanion
-
A New Route to γ-Arylidenebutyrolactones via a tandem carbopalladation-heterocyclisation sequence: a formal synthesis of U-68,215作者:Marcello Cavicchioli、Sylvie Decortiat、Didier Bouyssi、Jacques Goré、Geneviève BalmeDOI:10.1016/0040-4020(96)00637-0日期:1996.8obtained in good yields from pentynoic acids 3- or 5-substituted with an iodo-aryl moiety by palladium-catalyzed cyclization of their potassium carboxylates. Using this approach, an efficient new route to U-68,215 is described.
-
Allylic Chlorides. XX. Preparation and Properties of the 1-Bromo-3-chloro-1-propenes<sup>1</sup>作者:Lewis F. Hatch、Kenneth E. HarwellDOI:10.1021/ja01119a066日期:1953.12
表征谱图
-
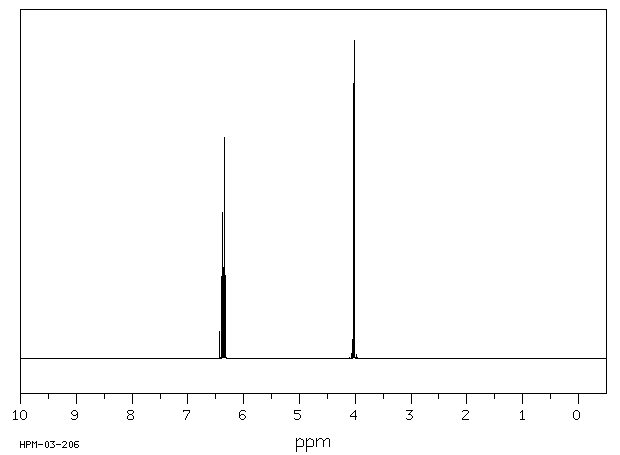
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-3-甲基-1,2,3,4-四氯-1-丁烯
顺式-1-溴-1-丙烯
顺式-1-氯-1-丁烯
顺式-1,3-二氯丙烯
顺式-1,2-二碘乙烯
顺式-1,2-二溴乙烯
顺式-1,2-二氟-1-氯乙烯
顺-氯丹
顺-九氯
顺-九氯
顺-1-溴-2-乙氧基乙烯
顺-1,2-二氯乙烯
顺-1,2,4-三氯-3-甲基-2-丁烯
顺,顺-1,2,3,4-四氯-1,3-丁二烯
除螨灵
锗烷,(1-溴-1,2-丙二烯基)三甲基-
锌,氯(三氟乙烯基)-
铜(1+),1,1,2-三氟乙烯
苯甲酸,4-[(1E)-2-[[(4-氯苯基)甲基]磺酰]乙烯基]-
苯并烯氟菌唑中间体
艾日布林-2碘
聚(乙烯-氯代三氟乙烯)
碳化镁碘化物
碘化乙烯
硫丹醇
硅烷,二氯(2-氯乙烯基)甲基-
硅烷,[2-(碘亚甲基)己基]三甲基-,(Z)-
甲碘乙烯
甲氧基全氟丁烷-反式-1,2-二氯乙烯1:1共沸物
甲基烯丙基溴化镁
甲基全氟-1-甲基-2-丙烯基醚
甲基全氟-1-丁基-1-丙烯基醚
甲基全氟-1-丁基-1-丙烯基醚
环丙烷,1,1-二氯-2-(3,3-二氯-2-甲基-2-丙烯基)-2,3,3-三甲基-
环丙烯,1,2-二氟-
特比萘芬杂质
溴西克林
溴甲基烯酮
溴环辛四烯
溴氯丙烯
溴代三氟代乙烯
溴亚甲基环己烷
溴乙烯
溴三碘乙烯
氰尿酰氟
氯磺酸三氟乙烯基酯
氯化聚乙烯
氯乙烯与异丁基乙烯醚共聚物
氯乙烯与三氯乙烯聚合物
氯乙烯-d3