吡啶-3-硫醇 | 16133-26-9
中文名称
吡啶-3-硫醇
中文别名
3-巯基吡啶
英文名称
pyridine-3-thiol
英文别名
3-mercaptopyridine;3-Pyridinethiol
CAS
16133-26-9
化学式
C5H5NS
mdl
MFCD00128263
分子量
111.167
InChiKey
FFWJHVGUAKWTKW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:78-80 °C(Solv: benzene (71-43-2); hexane (110-54-3))
-
沸点:194.0±13.0 °C(Predicted)
-
密度:1.166±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:13.9
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2933399090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-(甲基硫代)吡啶 3-methylsulfanylpyridine 18794-33-7 C6H7NS 125.194 —— 3-(ethylthio)pyridine 26891-59-8 C7H9NS 139.221 3-乙烯基硫基吡啶 3-vinylthiopyridine 140472-72-6 C7H7NS 137.205
反应信息
-
作为反应物:参考文献:名称:[EN] CARBACEPHEM ß-LACTAM ANTIBIOTICS
[FR] ANTIBIOTIQUES BÊTA-LACTAMES À CARBACÉPHÈME摘要:Carbacephem β-内酰胺抗生素具有以下化学结构(I)和(II),包括立体异构体、药用可接受的盐、酯和前药,其中Ar2、R1、R2和R3如本文所定义。这些化合物可用于治疗细菌感染,特别是由耐甲氧西林葡萄球菌属引起的感染。公开号:WO2009055696A1 -
作为产物:参考文献:名称:Barnes; Fatome; Esslemont, European Journal of Medicinal Chemistry, 1983, vol. 18, # 6, p. 515 - 519摘要:DOI:
文献信息
-
一种硫烯丙基碳酸酯类化合物及其制备方法
-
Copper(II)-Catalyzed Single-Step Synthesis of Aryl Thiols from Aryl Halides and 1,2-Ethanedithiol作者:Yajun Liu、Jihye Kim、Heesun Seo、Sunghyouk Park、Junghyun ChaeDOI:10.1002/adsc.201400941日期:2015.7.6single‐step synthesis of aryl thiols from aryl halides has been developed employing copper(II) catalyst and 1,2‐ethanedithiol. The key features are use of readily available reagents, a simple operation, and relatively mild reaction conditions. This new protocol shows a broad substrate scope with excellent functional group compatibility. A variety of aryl thiols are directly prepared from aryl halides in high
-
Iron-Catalyzed Cross-Coupling of Unactivated Secondary Alkyl Thio Ethers and Sulfones with Aryl Grignard Reagents作者:Scott E. Denmark、Alexander J. CresswellDOI:10.1021/jo402246h日期:2013.12.20ed cross-coupling are described. Initial studies focused on discerning the structural and electronic features of the organosulfur substrate that enable the challenging oxidative addition to the C(sp3)–S bond. Through extensive optimization efforts, an Fe(acac)3-catalyzed cross-coupling of unactivated alkyl aryl thio ethers with aryl Grignard reagents was realized in which a nitrogen “directing group”描述了未活化的脂肪族硫化合物作为过渡金属催化交叉偶联中的亲电试剂的首次系统研究。最初的研究侧重于识别有机硫底物的结构和电子特征,这些特征能够对 C(sp 3 )-S 键进行具有挑战性的氧化加成。通过广泛的优化工作,实现了未活化的烷基芳基硫醚与芳基格氏试剂的 Fe(acac) 3催化交叉偶联,其中硫醚 S-芳基部分上的氮“导向基团”起到了关键作用促进氧化加成步骤。此外,发现烷基苯砜是 Fe(acac) 3 中有效的亲电试剂。-催化与芳基格氏试剂的交叉偶联。对于后一类亲电试剂,对各种反应参数的彻底评估表明,过量的 TMEDA(8.0 当量)反应效率显着提高。优化的反应方案用于评估该方法在有机镁亲核试剂和砜亲电试剂方面的范围。
-
Metal-free oxidative coupling of alkyl chlorides with thiols: An efficient access to sulfoxides作者:Qian Liu、Xiaoqian Zhao、Feng Xu、Gaoqiang LiDOI:10.1016/j.tetlet.2019.151492日期:2020.2An efficient and step-economical access to sulfoxides from thiols and alkyl halides in the presence of I2O5 and DBU via direct oxidative couplings is described here. It is the first case that combined Williamson sulfide synthesis and subsequent sulfide oxidation into one step manipulation for sulfoxides preparation. This protocol features wide substrate scope, mild and metal-free conditons, the use
-
Design of thymidylate synthase inhibitors using protein crystal structures: the synthesis and biological evaluation of a novel class of 5-substituted quinazolinones作者:Stephen E. Webber、Ted M. Bleckman、John Attard、Judith G. Deal、Vinit Kathardekar、Katherine M. Welsh、Stephanie Webber、Cheryl A. Janson、David A. MatthewsDOI:10.1021/jm00058a010日期:1993.3The design, synthesis, and biological evaluation of a new class of inhibitors of thymidylate synthase (TS) is described. The molecular design was carried out by a repetitive crystallographic analysis of protein-ligand structures. At the onset of this project, we focused on the folate cofactor binding site of a high-resolution ternary crystal complex of Escherichia coli TS, 5'-fluorodeoxyuridylate (5-FdUMP)描述了新型胸苷酸合酶(TS)抑制剂的设计,合成和生物学评估。通过对蛋白质-配体结构的重复晶体学分析来进行分子设计。在该项目开始时,我们专注于大肠杆菌TS,5'-氟脱氧尿酸(5-FdUMP)和经典的含谷氨酸叶酸类似物的高分辨率三元晶体复合物的叶酸辅因子结合位点。一种新型化合物的初步三元晶体结构已成功解决。通过分析该初始复合物,进行了进一步的结构修饰,并开发了一系列活性5-(芳硫基)喹唑啉酮。合成策略基于各种芳基硫代阴离子置换喹唑啉酮5位上的卤素。测试化合物对纯化的大肠杆菌和/或人TS的抑制作用,并在体外测定针对三种肿瘤细胞系的细胞毒性。用几种抑制剂观察到了重要的胸腺嘧啶核苷保护作用,表明TS是细胞内活性位点。
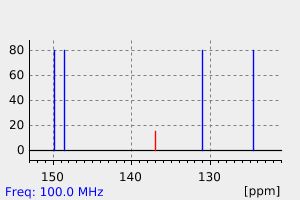
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-