(E)-1-(4-bromophenyl)-N-phenylmethanimine | 1613-97-4
中文名称
——
中文别名
——
英文名称
(E)-1-(4-bromophenyl)-N-phenylmethanimine
英文别名
N-[(1E)-(4-bromophenyl)methyliden]aniline;N-[(E)-(4-bromophenyl)methylidene]aniline;(E)-N-(4-bromobenzylidene)aniline;4-bromobenzylideneaniline;p-bromobenzylideneaniline
CAS
1613-97-4
化学式
C13H10BrN
mdl
——
分子量
260.133
InChiKey
MJSLSMOBYCYMIM-XNTDXEJSSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:15.0
-
可旋转键数:2.0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:12.36
-
氢给体数:0.0
-
氢受体数:1.0
上下游信息
反应信息
-
作为反应物:描述:(E)-1-(4-bromophenyl)-N-phenylmethanimine 在 二氢吡啶 作用下, 以 二氯甲烷 为溶剂, 反应 15.0h, 以93%的产率得到4-bromobenzylaniline参考文献:名称:Thiourea-Catalyzed Transfer Hydrogenation of Aldimines摘要:本信报告了以 1,4-二氢吡啶为氢源,通过氢键活化硫脲催化亚胺的转移加氢反应。在无酸和无金属的反应条件下,各种芳香族和脂肪族醛亚胺都能被还原生成相应的胺。DOI:10.1055/s-2007-980349
-
作为产物:描述:对溴苯甲醛 、 苯胺 在 lithium hydroxide monohydrate 作用下, 以 甲苯 为溶剂, 反应 4.0h, 以14%的产率得到(E)-1-(4-bromophenyl)-N-phenylmethanimine参考文献:名称:镍与钯在交叉偶联催化中的关系:底物配位对零价金属络合物的作用摘要:报道了使用竞争实验,鲁棒性筛选和密度泛函理论计算,比较了官能团对镍和钯催化的铃木-宫浦反应性能的影响的详细比较。镍可以与各种官能团相互作用,这在竞争性交叉偶联反应中表现为选择性。这些官能团在外源性添加剂上的存在对交叉偶联反应有影响,其范围从收率的轻微提高到反应完全停止。相比之下,钯与这些官能团的相互作用不足以引起交叉偶联反应的选择性。钯催化的交叉偶联反应的选择性主要由芳基卤化物的电子性质决定。DOI:10.1055/s-0039-1690045
文献信息
-
A Rapid and Additive-Free Ruthenium-Catalyzed Reductive Amination of Aromatic Aldehydes作者:Christian Kerner、Sascha-Dominic Straub、Y. Sun、Werner R. ThielDOI:10.1002/ejoc.201600515日期:2016.62-[2-(dimethylamino)pyrimidin-4-yl]pyridine} is highly reactive in catalyzing the reductive amination of aromatic aldehydes through in situ generated imines with 2-propanol as the hydrogen source following a transfer-hydrogenation mechanism. This transformation does not require any activating additives and is applicable to a broad variety of aldehydes.
-
Enantioselective and Regiodivergent Functionalization of<i>N-</i>Allylcarbamates by Mechanistically Divergent Multicatalysis作者:Edward Richmond、Ismat Ullah Khan、Joseph MoranDOI:10.1002/chem.201602792日期:2016.8.22A pair of mechanistically divergent multicatalytic reaction sequences has been developed consisting of nickel‐catalyzed isomerization of N‐allylcarbamates and subsequent phosphoric‐acid‐catalyzed enantioselective functionalization of the resulting intermediates. By appropriate selection of reaction partners, in situ generated imines and ene‐carbamates are mechanistically partitioned to yield opposing
-
Rapid synthesis of quinoline-4-carboxylic acid derivatives from arylimines and 2-substituted acrylates or acrylamides under indium(iii) chloride and microwave activations. Scope and limitations of the reaction作者:Dorothée Duvelleroy、Cécile Perrio、Olivier Parisel、Marie-Claire LasneDOI:10.1039/b509400c日期:——quinoline-4-carboxylic acid derivatives has been achieved by reaction of 2-methoxy acrylates or acrylamides with N-arylbenzaldimines in acetonitrile under InCl3 catalysis and microwave irradiation. Isolated yields up to 57% within 3 min have been obtained. The Lewis acid and the microwave activation appeared as crucial parameters for the reaction. The role of indium chloride and ytterbium triflate was specified
-
Molecular iodine-catalyzed one-pot synthesis of substituted quinolines from imines and aldehydes作者:Xu-Feng Lin、Sun-Liang Cui、Yan-Guang WangDOI:10.1016/j.tetlet.2006.02.136日期:2006.5A mild, efficient, and general method for the synthesis of substituted quinolines via a molecular iodine-catalyzed one-pot domino reaction of imines with enolizable aldehydes has been described.
-
Gold‐Catalyzed Bicyclic and [3+2]‐Annulations of Internal Propargyl Alcohols with Nitrones and Imines To Yield to Two Distinct Heterocycles作者:Sayaji Arjun More、Tzu‐Hsuan Chao、Mu‐Jeng Cheng、Rai‐Shung LiuDOI:10.1002/adsc.202001119日期:2021.1.194‐a]indoles from 1‐oxo‐3‐yn‐4‐ols and nitrones is described; this new bicyclic annulation presents the first examples that internal alkynes can react with nitrones to undergo an oxoarylation route. DFT calculations indicate a [3,3]‐sigmatropic shift of initial alkenylgold intermediates to elude the intermediacy of gold carbenes. We also developed new [3+2]‐annulations of the same 1‐oxo‐3‐yn‐4‐ols with imines
表征谱图
-
氢谱1HNMR
-
质谱MS
-
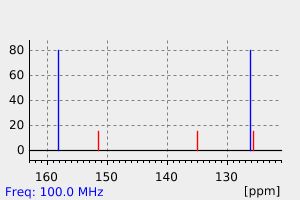
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫