4-羟基苯甲酸正己酯 | 1083-27-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:53°C
-
沸点:171°C/2mmHg(lit.)
-
密度:1.0850 (rough estimate)
-
溶解度:溶于甲醇
-
LogP:4.350
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:16
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.461
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
RTECS号:DH2204000
-
海关编码:2918290000
-
危险性防范说明:P261,P264,P272,P280,P302+P352+P333+P313+P363,P305+P351+P338+P337+P313,P501
-
危险性描述:H315,H317,H319
-
储存条件:室温且干燥环境下使用。
SDS
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Hexyl 4-Hydroxybenzoate
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
Skin sensitization Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
May cause an allergic skin reaction
Precautionary statements:
Avoid breathing dust/fume/gas/mist/vapours/spray.
[Prevention]
Contaminated work clothing should not be allowed out of the workplace.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation or rash occurs: Get medical advice/attention.
Wash contaminated clothing before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Hexyl 4-Hydroxybenzoate
Percent: >98.0%(LC)(T)
1083-27-8
CAS Number:
Synonyms: Hexylparaben , 4-Hydroxybenzoic Acid Hexyl Ester
Chemical Formula: C13H18O3
Hexyl 4-Hydroxybenzoate
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
No data available
Odour:
Hexyl 4-Hydroxybenzoate
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
pH: No data available
Melting point/freezing point:53°C
Boiling point/range: 171°C/0.3kPa
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Log Pow: 4.35
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: scu-mus LD50:3400 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
RTECS Number: DH2204000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: 4.35
No data available
Soil adsorption (Koc):
1.51 x 10-3
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Hexyl 4-Hydroxybenzoate
Section 14. TRANSPORT INFORMATION
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:4-羟基苯甲酸正己酯 在 bis-triphenylphosphine-palladium(II) chloride 、 copper(l) iodide 、 4-吡咯烷基吡啶 三乙胺 、 N,N'-二环己基碳二亚胺 作用下, 以 乙醚 为溶剂, 生成 4'-(n-Hexyloxycarbonyl)phenyl 4''-<(4-(S)-2'-methylbutoxy-2,3,5,6-tetrafluorophenyl)ethynyl>benzoate参考文献:名称:Xu, Yuelian; Chen, Qi; Wen, Jianxun, Molecular Crystals and Liquid Crystals Science and Technology, Section A: Molecular Crystals and Liquid Crystals, 1994, vol. 241, p. 243 - 248摘要:DOI:
-
作为产物:描述:参考文献:名称:Olivier, Recueil des Travaux Chimiques des Pays-Bas, 1936, vol. 55, p. 1031摘要:DOI:
文献信息
-
Synthesis and biochemical evaluation of some novel benzoic acid based esters as potential inhibitors of oestrone sulphatase作者:Caroline Owen、Karen James、Luther Sampson、Sabbir AhmedDOI:10.1211/002235702568日期:2010.2.18
Abstract Oestrone sulphatase is an important target in the fight against hormone-dependent breast cancer. In an effort to investigate the reported definitive pharmacophore for oestrone sulphatase and continue our search for potent inhibitors of this enzyme, we have undertaken extensive synthesis, biochemical evaluation and physicochemical property determination of a range of benzoic acid based esters. Here, we report the initial results of our study into a series of straight chain alkyl esters of 4-sulphonylbenzoic acid. Using these compounds, we have investigated the involvement of two physicochemical properties, namely logP and pKa. The results of this study show that there was a strong correlation between the inhibitory activity and the logP of the parent compound. Within the series of compounds studied, hydrophobicity appears to be a more important factor than pKa in determining the overall inhibitory activity. In a previous report, we showed that pKa plays an important role in stabilizing the phenoxide ion resulting from the hydrolysis of the sulphamate group. Here, we propose that although pKa is an important factor in determining the overall inhibitory activity when a wide range of compounds are considered, both hydrophobicity and pKa need to be considered in the design of potential inhibitors of oestrone sulphatase.
醇酮硫酸酶是对抗激素依赖性乳腺癌的重要靶点。为了研究已报道的醇酮硫酸酶的明确药效团并继续寻找这种酶的有效抑制剂,我们进行了大量的苯甲酸酯类化合物的合成、生化评价和理化性质测定。在这里,我们报告了我们对一系列直链烷基苯磺酸酯的研究初步结果。使用这些化合物,我们研究了两种理化性质,即logP和pKa的参与。这项研究结果显示,抑制活性与母体化合物的logP之间存在强烈相关性。在研究的化合物系列中,疏水性似乎比pKa更重要,决定了整体的抑制活性。在先前的报告中,我们表明pKa在稳定由磺酸酯基水解产生的苯酚根离子中起重要作用。在这里,我们提出,尽管pKa在考虑广泛的化合物时在确定整体抑制活性中起重要作用,但在设计潜在的醇酮硫酸酶抑制剂时,需要同时考虑疏水性和pKa。 -
Metabolism and Pharmacokinetics of Oxazaphosphorines作者:Alan V. Boddy、S. Murray YuleDOI:10.2165/00003088-200038040-00001日期:2000.4The 2 most commonly used oxazaphosphorines are cyclophosphamide and ifosfamide, although other bifunctional mustard analogues continue to be investigated. The pharmacology of these agents is determined by their metabolism, since the parent drug is relatively inactive. For cyclophosphamide, elimination of the parent compound is by activation to the 4-hydroxy metabolite, although other minor pathways of inactivation also play a role. Ifosfamide is inactivated to a greater degree by dechloroethylation reactions. More robust assay methods for the 4-hydroxy metabolites may reveal more about the clinical pharmacology of these drugs, but at present the best pharmacodynamic data indicate an inverse relationship between plasma concentration of parent drug and either toxicity or antitumour effect. The metabolism of cyclophosphamide is of particular relevance in the application of high dose chemotherapy. The activation pathway of metabolism is saturable, such that at higher doses (greater than 2 to 4 g/m2) a greater proportion of the drug is eliminated as inactive metabolites. However, both cyclophosphamide and ifosfamide also act to induce their own metabolism. Since most high dose regimens require a continuous infusion or divided doses over several days, saturation of metabolism may be compensated for, in part, by auto-induction. Although a quantitative distinction may be made between the cytochrome P450 isoforms responsible for the activating 4-hydroxylation reaction and those which mediate the dechloroethylation reactions, selective induction of the activation pathway, or inhibition of the inactivating pathway, has not been demonstrated clinically. Mathematical models to describe and predict the relative contributions of saturation and autoinduction to the net activation of cyclophosphamide have been developed. However, these require careful validation and may not be applicable outside the exact regimen in which they were derived. A further complication is the chiral nature of these 2 drugs, with some suggestion that one enantiomer may have a favourable profile of metabolism over the other. That the oxazaphosphorines continue to be the subject of intensive investigation over 30 years after their introduction into clinical practice is partly because of their antitumour activity. Further advances in analytical and molecular pharmacological techniques may further optimise their use and allow rational design of more selective analogues.最常用的两种噁唑磷酰胺类药物是环磷酰胺和异环磷酰胺,尽管其他双功能烷化剂仍在研究中。这些药物的药理学特性由其代谢途径决定,因为母体药物相对不活跃。环磷酰胺通过激活生成4-羟基代谢物来消除母体化合物,尽管其他次要的失活途径也起作用。异环磷酰胺通过脱氯乙基化反应更大幅度地失活。更强大的4-羟基代谢物检测方法可能揭示这些药物的临床药理学更多信息,但目前最佳药效学数据显示,母体药物血浆浓度的逆向关系与毒性或抗肿瘤效应相关。环磷酰胺的代谢在应用高剂量化疗时尤为相关。代谢激活途径是可饱和的,因此在高剂量(大于2至4 g/m²)下,药物以失活代谢物的形式排出的比例更大。然而,环磷酰胺和异环磷酰胺都能诱导自身代谢。由于大多数高剂量方案需要连续输注或几天的分次给药,代谢饱和可通过自诱导部分得到补偿。尽管可以对负责激活4-羟基化反应的细胞色素P450同工酶和介导脱氯乙基化反应的同工酶进行定量区分,但临床上尚未证明选择性诱导激活途径或抑制失活途径的能力。描述和预测饱和和自诱导对环磷酰胺净激活相对贡献的数学模型已开发出来。然而,这些模型需要仔细验证,并且可能不适用于它们所衍生的给药方案之外。另一个复杂之处在于这两种药物的手性本质,有迹象表明一种对映体可能比另一种具有更有利的代谢特征。由于其抗肿瘤活性,噁唑磷酰胺类药物在引入临床实践30多年后,仍然是广泛研究的主题。进一步发展分析和分子药理学技术可能进一步优化它们的使用,并允许设计更具选择性的类似物。
-
Inhibition of estrone sulfatase (ES) by alkyl and cycloalkyl ester derivatives of 4-[(aminosulfonyl)oxy] benzoic acid作者:Chirag K. Patel、Caroline P. Owen、Sabbir AhmedDOI:10.1016/j.bmcl.2003.11.067日期:2004.2In our search for potent inhibitors of the enzyme estrone sulfatase (ES), we have undertaken the synthesis and biochemical evaluation of a range of esters of 4-[(aminosulfonyl)oxy] benzoic acid. The results of the study show that the synthesised compounds possess potent inhibitory activity, indeed the cyclooctyl derivative was found to be more potent than 667-COUMATE, which is currently undergoing
-
Hexyl triazabutadiene as a potent alkylating agent作者:Diana C. Knyazeva、Flora W. Kimani、Jean-Laurent Blanche、John C. JewettDOI:10.1016/j.tetlet.2017.05.056日期:2017.7Alkyl diazonium ions are among the most reactive alkylating agents in the synthetic chemists’ arsenal. That said, there are precious few methods by which one can selectively and safely utilize this chemistry. Herein, we show the use of a bench stable hexyl triazabutadiene as a source of reactive diazonium ions that undergo substitution chemistry with weak nucleophiles, such as carboxylates and even
-
HIGHLY PHOTO-STABLE BIS-TRIAZOLE FLUOROPHORES申请人:NITTO DENKO CORPORATION公开号:US20180002337A1公开(公告)日:2018-01-04This disclosure is related to photo-stable chromophores which are useful in various applications. Chromophores disclosed herein include a bis-triazole core, two electron-donors at C-4 and C-8, and two groups derived from pentaerythritol (R═OR 5 ) or 1,1,1-tris(hydroxymethyl)methane (R═H) at N-2 and N-6. Such structures have been proven to have greater then five times higher photo-stability than their analogs with simpler alkyl groups at N-2 and N-6.
表征谱图
-
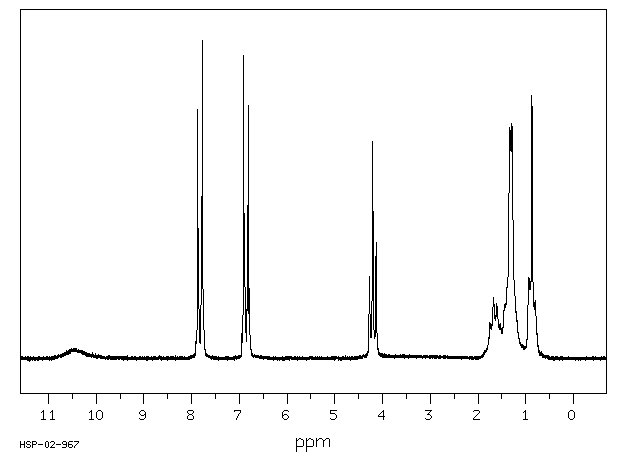
氢谱1HNMR
-
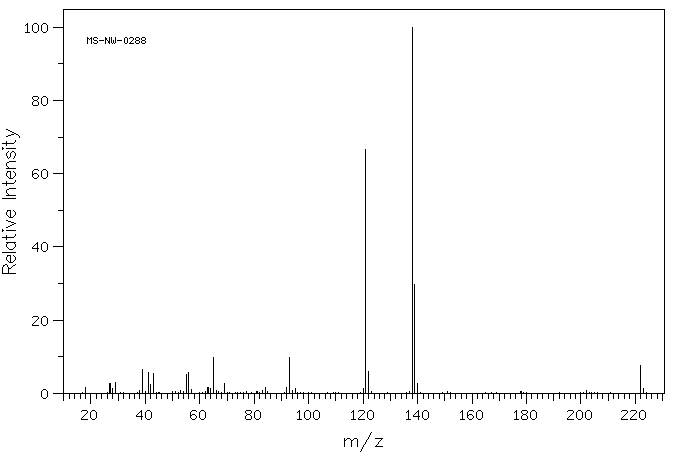
质谱MS
-
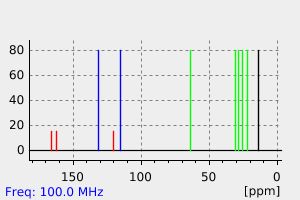
碳谱13CNMR
-
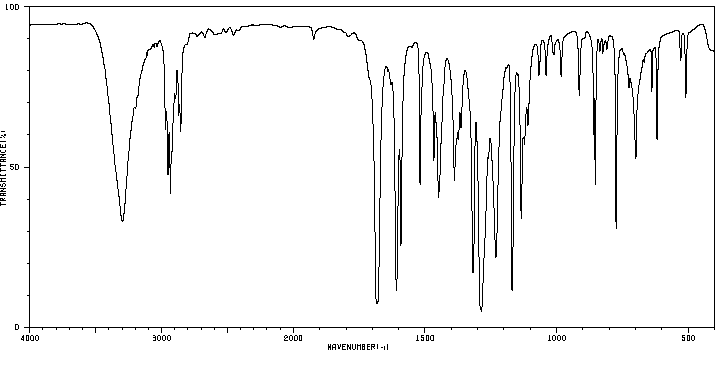
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息