2-氟蒽醌 | 572-84-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:196 °C
-
沸点:397.2±31.0 °C(Predicted)
-
密度:1.385±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:17
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-fluoro-3-methylanthraquinone 523-17-1 C15H9FO2 240.234 2-(4-氟苯酰基)苯甲酸 2-(4-fluorobenzoyl)benzoic acid 7649-92-5 C14H9FO3 244.222 2-氯蒽醌 2-chloroanthracene-9,10-dione 131-09-9 C14H7ClO2 242.661 2-氨基蒽醌 2-aminoanthraquinone 117-79-3 C14H9NO2 223.231 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-amino-6-fluoro-anthraquinone 569-09-5 C14H8FNO2 241.221 2-氨基蒽醌 2-aminoanthraquinone 117-79-3 C14H9NO2 223.231
反应信息
-
作为反应物:描述:参考文献:名称:THE PREPARATION OF SOME FLUORO-NITRO-ANTHRAQUINONES AND THEIR REDUCTION TO AMINO-FLUORO-ANTHRAQUINONES摘要:不可用DOI:10.1139/v63-459
-
作为产物:描述:参考文献:名称:Hahn; Reid, Journal of the American Chemical Society, 1924, vol. 46, p. 1647摘要:DOI:
文献信息
-
Extended Bis(anthraoxa)quinodimethanes with Nine and Ten Consecutively Fused Six-Membered Rings: Neutral Diradicaloids and Charged Diradical Dianions/Dications作者:Shaoqiang Dong、Tullimilli Y. Gopalakrishna、Yi Han、Hoa Phan、Tao Tao、Yong Ni、Gang Liu、Chunyan ChiDOI:10.1021/jacs.8b10279日期:2019.1.9respective nonacene and decacene. The dication ABA2+ and dianion ABA2- are open-shell singlet diradicaloids, while the longer dication ANA-TFA2+ and dianion ANA2- have closed-shell ground state, which can be explained by the different intramolecular Coulomb interactions. Both dianions have a bent backbone and can be considered as an isoelectronic structure of the tetraanion of nonacene and decacene, respectively
-
Stable Simple Enols. Resolution of Chiral 1-[9‘-(2‘-Fluoroanthryl)]-2,2-Dimesitylethenol. A Different Racemization Mechanism for the Enol and its Acetate<sup>1</sup>作者:Elimelech Rochlin、Zvi RappoportDOI:10.1021/jo020497q日期:2003.1.1expected for a rotational betabeta'-2-ring flip process in a vinyl propeller and the racemization is unaffected by added TFA, Et3N, and EtOD. Although 3 racemizes almost 350 times faster, the racemization is catalyzed by TFA and shows bell-shape catalysis by Et3N and a KIE in a partially deuteriated solvent. From this and the DNMR data, it is concluded that 3 does not racemize via a rotational betabeta'-2-ring手性1- [9'-(2'-甲氧基蒽基)]-2,2-二甲基间苯三酚(2),1- [9'-(2'-氟蒽基)]-2,2-二甲基间苯三酚(3)和1-合成了[9'-(2'-氟蒽基)]-2,2-二聚乙烯乙酸乙烯酯(4),研究了它们在C6D5NO2中的DNMR行为。将3和4在直链淀粉三(3,5-二甲基苯基氨基甲酸酯)HPLC柱上拆分为它们的对映异构体。醋酸盐4在DeltaG(e)(++),DeltaH(e)(++)和DeltaS(e)(++)值分别为26.2、27.6 kcal mol(-)(1)和4.3的溶液中缓慢消旋分别对乙烯基螺旋桨中的旋转betabeta'-2-环翻转过程而言是预期的eu,外消旋化不受添加的TFA,Et3N和EtOD的影响。尽管3的消旋速度快了350倍,但消旋作用由TFA催化,在部分氘代的溶剂中由Et3N和KIE呈钟形催化。从该数据和DNMR数据得出的结论是3不会通过旋转的betabeta'-2-ring
-
Products of the condensation reaction of 2-(4-fluorobenzoyl)benzoic acid with m-cresol作者:J. Gronowska、A. KalinowskaDOI:10.1016/s0022-1139(00)80107-4日期:1993.4The preparation and structural elucidation of the new isomeric 3-(4-fluorophenyl)-3-(hydroxymethyl)phthalidesmade from m-cresol and 2-(4-fluorobenzoyl)benzoic acid aredescribed.
-
Aromatic compounds containing fluorine and a process of preparing them申请人:HOECHST AG公开号:US02891074A1公开(公告)日:1959-06-16
A process for the manufacture of aromatic compounds containing fluorine comprises reacting an aromatic compound containing one or more chlorine atoms bound to the nucleus and, in addition to chlorine, at least two strongly electro-negative substituents, at least one of which is, in the case of compounds of the benzene or naphthalene series, in ortho- or para - position (for naphthalene compounds "ortho-" includes 1 : 8-position and "para-" includes 2 : 5 position) to a chlorine atom, with dry potassium fluoride at a raised temperature and in absence of a solvent. The electro-negative substituents may be nitro, sulphonamido, sulpho-ester, carboxy-ester, carbamido, carboxyanhydride or trifluoromethyl groups or quinone oxygen (in the case of anthraquinones the chlorine atom or atoms may be in either the a - or b -positions). The process may be carried out by heating in a closed vessel to a temperature between 175 DEG and 275 DEG C., using appropriate quantities of potassium fluoride to partially or wholly replace chlorine atoms by fluorine atoms. New compounds prepared by the process are dinitro-chloro-difluorobenzenes from 1 : 3-dinitro-2 : 5 : 6-trichlorobenzene and from 1 : 3 - dinitro - 2 : 4 : 6 - trichlorobenzene; 1 : 3 - dinitro - 4 - fluoro - 5 - trifluoromethylbenzene from 1 : 3 - dinitro - 4 - chloro - 5 - trifluoromethyl - benzene; 2 - chloro - 3 - fluoronaphthoquinone from 2 : 3-dichloro-naphthoquinone; 1 - fluoroanthraquinone from 1-chloroanthraquinone; 1 : 8 - difluoroanthraquinone from 1 : 8 - dichloroanthraquinone; 2 : 5 - difluoro - 3 : 6 - dichloro - 1 : 4 - benzoquinone and 2 - fluoro - 3 : 5 : 6 - trichloro-1 : 4 - benzoquinon from chloranil; and 1-fluoro - 2 - nitrobenzene - 4 - sulphonic acid diethylamide from 1 - chloro - 2 - nitrobenzene-4 - sulphonic acid diethylamide. Other compounds which may be prepared by the process include 1 : 3 - dinitro - 4 : 6 - difluorobenzene and 1 : 3 - dinitro - 4 - chloro - 6 - fluorobenzene from 1 : 3 - dinitro - 4 : 6 - dichlorobenzene; 3-nitro - 4 - fluoro - benzoic acid - ethyl ester from 3 - nitro - 4 - chloro - benzoic acid ethyl ester; 3 - nitro - 6 - fluoro - benzoic acid methyl ester from 3-nitro-6-chloro-benzoic acid methyl ester; 4-fluoro-phthalic anhydride from 4-chloro-phthalic anhydride; and 2-fluoroanthraquinone from 2-chloroanthraquinone.
一种制备含氟芳香化合物的方法包括将一种含有一个或多个氯原子结合到核上的芳香化合物与氟化钾在升高的温度下反应,且除氯外,还至少有两个强电负取代基,其中至少一个在苯或萘系列化合物中位于邻位或对位(对于萘化合物,“邻-”包括1:8位,“对-”包括2:5位)至氯原子。这些电负取代基可以是硝基、磺胺基、磺酸酯、羧酯、氨基、羧酸酐或三氟甲基基团或醌氧(在蒽醌的情况下,氯原子可以位于a-或b-位置)。该过程可通过在封闭容器中加热至175摄氏度至275摄氏度的温度,使用适量的氟化钾部分或完全用氟原子取代氯原子来进行。该过程制备的新化合物包括1:3-二硝基-氯二氟苯、1:3-二硝基-4-氟-5-三氟甲基苯、2-氯-3-氟萘醌、1-氟蒽醌、1:8-二氟蒽醌、2:5-二氟-3:6-二氯-1:4-苯醌、2-氟-3:5:6-三氯-1:4-苯醌、1-氟-2-硝基苯-4-磺酸二乙酰胺等。其他可能通过该方法制备的化合物包括1:3-二硝基-4:6-二氟苯、1:3-二硝基-4-氯-6-氟苯、3-硝基-4-氟苯甲酸乙酯、3-硝基-6-氟苯甲酸甲酯、4-氟邻苯二酐、2-氟蒽醌等。 -
Access to Diarylmethanols by Wittig Rearrangement of <i>ortho-</i>, <i>meta-</i>, and <i>para-</i>Benzyloxy-<i>N</i>-Butylbenzamides作者:R. Alan Aitken、Andrew D. Harper、Ryan A. Inwood、Alexandra M. Z. SlawinDOI:10.1021/acs.joc.1c03160日期:2022.4.1promoter of the [1,2]-Wittig rearrangement of aryl benzyl ethers and thus allow the two-step synthesis of isomerically pure substituted diarylmethanols starting from simple hydroxybenzoic acid derivatives. The method is compatible with a wide range of functional groups including methyl, methoxy, and fluoro, although not with nitro and, unexpectedly, is applicable to meta as well as ortho and para isomeric
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
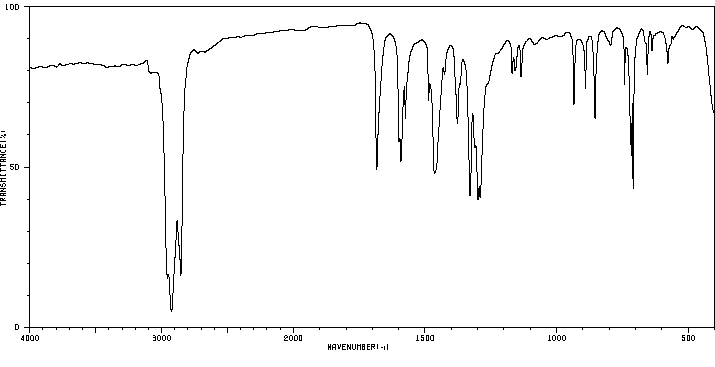
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息