4-(乙硫基)甲苯 | 622-63-9
中文名称
4-(乙硫基)甲苯
中文别名
4-乙硫基甲苯
英文名称
ethyl p-tolyl sulfide
英文别名
ethyl(p-tolyl)sulfane;ethyl 4-methylphenyl sulfide;1-(ethylthio)-4-methylbenzene;4-methylphenyl ethyl sulfide;4-(Ethylthio)toluene;1-ethylsulfanyl-4-methylbenzene
CAS
622-63-9
化学式
C9H12S
mdl
MFCD00093776
分子量
152.26
InChiKey
TXMFDSUMJLQLGB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-45°C (estimate)
-
沸点:224.76°C (estimate)
-
密度:0.9956
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:10
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2930909090
-
储存条件:室温且干燥
SDS
Version 1.0
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name ETHYL P-TOLYL SULFIDE - 50 MG
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
Not hazardous according to Directive 67/548/EEC.
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
ETHYL P-TOLYL SULFIDE 622-63-9 None None
Formula C9H12S
Molecular Weight 152,2600 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of contact, immediately wash skin with soap and copious
amounts of water.
AFTER EYE CONTACT
In case of contact, immediately flush eyes with copious amounts
of water for at least 15 minutes.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: For small (incipient) fires, use media such as
ALDRICH www.molbase.com
"alcohol" foam, dry chemical, or carbon dioxide. For large
fires, apply water from as far as possible. Use very large
quantities (flooding) of water applied as a mist or spray; solid
streams of water may be ineffective. Cool all affected
containers with flooding quantities of water.
SPECIAL RISKS
Specific Hazard(s): Combustible liquid. Emits toxic fumes under
fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PERSONAL PRECAUTION PROCEDURES TO BE FOLLOWED IN CASE OF LEAK OR SPILL
Evacuate area.
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear respirator, chemical safety goggles, rubber boots, and
heavy rubber gloves.
METHODS FOR CLEANING UP
Cover with dry-lime, sand, or soda ash. Place in covered
containers using non-sparking tools and transport outdoors.
Ventilate area and wash spill site after material pickup is
complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid breathing vapor. Avoid
contact with eyes, skin, and clothing. Avoid prolonged or
repeated exposure.
STORAGE
Conditions of Storage: Keep tightly closed. Keep away from heat
and open flame.
SPECIAL REQUIREMENTS: Stench.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash contaminated clothing before reuse. Wash thoroughly after
handling.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Where risk assessment shows air-purifying respirators
are appropriate use a full-face respirator with multi-purpose
combination (US) or type ABEK (EN 14387) respirator cartridges as
a backup to engineering controls. If the respirator is the sole
means of protection, use a full-face supplied air respirator.
Hand Protection: Compatible chemical-resistant gloves.
ALDRICH www.molbase.com
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
Appearance Physical State: Liquid
Odor: Stench.
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Flash Point 76,700. - 87,800 °C.
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient Log Kow: 3,748
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Sulfur oxides.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: May be harmful if absorbed through the skin.
Eye Contact: May cause eye irritation.
Inhalation: May be harmful if inhaled. Material may be
irritating to mucous membranes and upper respiratory tract.
Ingestion: May be harmful if swallowed.
ALDRICH www.molbase.com
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. This combustible material may be burned in a
chemical incinerator equipped with an afterburner and scrubber.
Observe all federal, state, and local environmental regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
UN#: 3334
Class: 9
Proper Shipping Name: Aviation Regulated Liquid, N.O.S.
Inhalation Packing Group I: No
Technical Name: Required
15 - Regulatory Information
Not hazardous according to Directive 67/548/EEC.
Caution: Substance not yet fully tested (EU).
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name ETHYL P-TOLYL SULFIDE - 50 MG
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
Not hazardous according to Directive 67/548/EEC.
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
ETHYL P-TOLYL SULFIDE 622-63-9 None None
Formula C9H12S
Molecular Weight 152,2600 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of contact, immediately wash skin with soap and copious
amounts of water.
AFTER EYE CONTACT
In case of contact, immediately flush eyes with copious amounts
of water for at least 15 minutes.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: For small (incipient) fires, use media such as
ALDRICH www.molbase.com
"alcohol" foam, dry chemical, or carbon dioxide. For large
fires, apply water from as far as possible. Use very large
quantities (flooding) of water applied as a mist or spray; solid
streams of water may be ineffective. Cool all affected
containers with flooding quantities of water.
SPECIAL RISKS
Specific Hazard(s): Combustible liquid. Emits toxic fumes under
fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PERSONAL PRECAUTION PROCEDURES TO BE FOLLOWED IN CASE OF LEAK OR SPILL
Evacuate area.
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear respirator, chemical safety goggles, rubber boots, and
heavy rubber gloves.
METHODS FOR CLEANING UP
Cover with dry-lime, sand, or soda ash. Place in covered
containers using non-sparking tools and transport outdoors.
Ventilate area and wash spill site after material pickup is
complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid breathing vapor. Avoid
contact with eyes, skin, and clothing. Avoid prolonged or
repeated exposure.
STORAGE
Conditions of Storage: Keep tightly closed. Keep away from heat
and open flame.
SPECIAL REQUIREMENTS: Stench.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash contaminated clothing before reuse. Wash thoroughly after
handling.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Where risk assessment shows air-purifying respirators
are appropriate use a full-face respirator with multi-purpose
combination (US) or type ABEK (EN 14387) respirator cartridges as
a backup to engineering controls. If the respirator is the sole
means of protection, use a full-face supplied air respirator.
Hand Protection: Compatible chemical-resistant gloves.
ALDRICH www.molbase.com
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
Appearance Physical State: Liquid
Odor: Stench.
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Flash Point 76,700. - 87,800 °C.
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient Log Kow: 3,748
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Sulfur oxides.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: May be harmful if absorbed through the skin.
Eye Contact: May cause eye irritation.
Inhalation: May be harmful if inhaled. Material may be
irritating to mucous membranes and upper respiratory tract.
Ingestion: May be harmful if swallowed.
ALDRICH www.molbase.com
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. This combustible material may be burned in a
chemical incinerator equipped with an afterburner and scrubber.
Observe all federal, state, and local environmental regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
UN#: 3334
Class: 9
Proper Shipping Name: Aviation Regulated Liquid, N.O.S.
Inhalation Packing Group I: No
Technical Name: Required
15 - Regulatory Information
Not hazardous according to Directive 67/548/EEC.
Caution: Substance not yet fully tested (EU).
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1-iodo-2-(4-methylbenzenethio)ethane 57023-00-4 C9H11IS 278.157 1-[(2-氯乙基)硫代]-4-甲苯 (2-chloro-ethyl)-p-tolyl sulfide 20761-71-1 C9H11ClS 186.705 4-甲基茴香硫醚 4-methylphenyl methylsulfide 623-13-2 C8H10S 138.233 —— 3-[(4-methylphenyl)sulfanyl]-1-propanol 3147-28-2 C10H14OS 182.287 对甲苯基乙基亚砜 ethyl (p-tolyl)sulfoxide 6378-07-0 C9H12OS 168.26 苯乙硫醚 Ethyl phenyl sulfide 622-38-8 C8H10S 138.233 4-甲苯硫酚 para-thiocresol 106-45-6 C7H8S 124.207 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-ethyl-4-(ethylthio)benzene 22905-10-8 C10H14S 166.287 —— 1-ethyl-4-<(methylethyl)thio>benzene —— C11H16S 180.314 —— (+)-(R)-ethyl p-tolyl sulfoxide 1519-40-0 C9H12OS 168.26 —— (S)-1-(ethylsulfinyl)-4-methylbenzene 62961-00-6 C9H12OS 168.26 对甲苯基乙基亚砜 ethyl (p-tolyl)sulfoxide 6378-07-0 C9H12OS 168.26 苯乙硫醚 Ethyl phenyl sulfide 622-38-8 C8H10S 138.233 —— 2,4-dimethyl-1-(ethylthio)benzene 107832-66-6 C10H14S 166.287 4-甲苯硫酚 para-thiocresol 106-45-6 C7H8S 124.207 1-乙基磺酰基-4-甲基-苯 ethyl p-tolyl sulfone 7569-34-8 C9H12O2S 184.259 —— 1-chloroethyl p-tolyl sulfoxide 31350-93-3 C9H11ClOS 202.705 1-氯乙基对甲苯基亚砜 (-)-(R)-1-chloroethyl p-tolyl sulfoxide 31350-93-3 C9H11ClOS 202.705 —— 4-(methylsulfanyl)phenyl 4-tolyl sulfide —— C14H14S2 246.397 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:化学选择性一锅法N-和O-转移从硫化物合成NH-磺胺嘧啶摘要:通过在同一反应中进行O和NH转移,可以从硫化物直接合成NH-亚砜肟类,并具有完全的选择性。该反应由双乙酰氧基碘苯在简单的条件下介导。DOI:10.1039/c6cc08891k
-
作为产物:描述:参考文献:名称:分子内反应。第12部分。环的大小和离去基团对碳负离子分子间和分子内亲核取代的影响摘要:在芳基ω-卤代烷基酮与碱环合成芳基环烷基酮的过程中,环丙烷的形成速度比环戊烷快23 000倍。氢-氘交换实验和三元环形成的极低的溴化氯化物比率(1.9)与确定酮的去质子速率的反应是一致的。相比之下,在五元环形成中,与羰基相邻的氢-氘交换发生的速度比环化快得多,并且氯-溴化物的比率在“ 99”处为“正常”。在由芳基磺酰基丙基芳烃磺酸酯形成芳基磺酰基环丙烷时,Hammettρ值表示离去基团为+1.7,而对于双磺酰基稳定的碳负离子的分子间取代为+1.2。试图获得ρ LG竞争的分子间反应使五元环形成的值受挫。在先前关于通过分子内亲核取代形成环的工作的背景下讨论了该结果。DOI:10.1039/p29820000579
文献信息
-
[EN] HETEROCYCLIC COMPOUNDS FOR THE TREATMENT OF STRESS-RELATED CONDITIONS<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES POUR LE TRAITEMENT D'ÉTATS LIÉS AU STRESS申请人:OTSUKA PHARMA CO LTD公开号:WO2010137738A1公开(公告)日:2010-12-02The present invention provides a novel heterocyclic compound. A heterocyclic compound represented by general formula (1) wherein, R1 and R2, each independently represent hydrogen; a phenyl lower alkyl group that may have a substituent(s) selected from the group consisting of a lower alkyl group and the like on a benzene ring and/or a lower alkyl group; or a cyclo C3-C8 alkyl lower alkyl group; or the like; R3 represents a lower alkynyl group or the like; R4 represents a phenyl group that may have a substituent(s) selected from the group consisting of a 1,3,4-oxadiazolyl group that may have e.g., halogen or a heterocyclic group selected from pyridyl group and the like; the heterocyclic group may have at least one substituent(s) selected from a lower alkoxy group and the like or a salt thereof.
-
[EN] CHROMAN-4-ONE DERIVATIVES FOR THE TREATMENT AND PROPHYLAXIS OF HEPATITIS B VIRUS INFECTION<br/>[FR] DÉRIVÉS DE CHROMAN-4-ONE POUR LE TRAITEMENT ET LA PROPHYLAXIE D'UNE INFECTION PAR LE VIRUS DE L'HÉPATITE B申请人:HOFFMANN LA ROCHE公开号:WO2020120642A1公开(公告)日:2020-06-18The present invention provides compounds having the general formula (I) wherein R1 to R10, Gi, G2, X and m are as described herein, compositions including the compounds and methods of using the compounds for treating hepatitis B.本发明提供具有一般式(I)的化合物,其中R1至R10、Gi、G2、X和m如本文所述,包括这些化合物的组合物以及使用这些化合物治疗乙型肝炎的方法。
-
Highly atom-economic, catalyst- and solvent-free oxidation of sulfides into sulfones using 30% aqueous H2O2作者:Marjan JerebDOI:10.1039/c2gc36073j日期:——Highly atom-efficient oxidation of sulfides into sulfones under solvent- and catalyst-free reaction conditions using a 30% aqueous solution of H2O2 at 75 °C is reported. A structurally diverse set of phenyl alkyl-, phenyl benzyl-, benzyl alkyl-, dialkyl-, heteroaryl alkyl- and cyclic sulfides were transformed into sulfones regardless of the aggregate state and electronic nature of the substituents原子效率高 氧化作用 的 硫化物 进入 砜类 在下面 溶剂- 和 催化剂报道了在75℃下使用30%的H 2 O 2水溶液的无反应条件。结构上多样化的一组苯基 烷基-, 苯基 苄基-, 苄基 烷基-,二烷基-, 杂芳基 烷基-和循环 硫化物 被转化为 砜类与取代基的聚集状态和电子性质无关。尽管整个工作过程中反应混合物均不均匀,但没有发现搅拌困难和反应进展的问题。在许多情况下,仅使用过量10 mol%的H 2 O 2,因此大大提高了该方法的高原子经济性。一些固体基材需要可变过量的过氧化氢; 但是,反应是严格进行的,没有有机物溶剂。事实证明,这种转变适合液体和固体放大硫化物。此外,隔离和纯化 的原油产品可以仅用 过滤 和 结晶。
-
Modulation of photochemical oxidation of thioethers to sulfoxides or sulfones using an aromatic ketone as the photocatalyst作者:Bin Zhao、Gerald B. Hammond、Bo XuDOI:10.1016/j.tetlet.2021.153376日期:2021.10We have developed an eco-friendly and chemo-selective photocatalytic synthesis of sulfoxides or sulfones via oxidation of sulfides (thioethers) at ambient temperature using air or O2 as the oxidant. An inexpensive thioxanthone was used as the photocatalyst. Our method offers excellent chemical yields and good functional group tolerance. The hydrogen bonding between hexafluoro-2-propanol (HFIP) and
-
Copper-catalyzed Ullmann coupling under ligand- and additive-free conditions. Part 2: S-Arylation of thiols with aryl iodides作者:Pongchart Buranaprasertsuk、Joyce Wei Wei Chang、Warinthorn Chavasiri、Philip Wai Hong ChanDOI:10.1016/j.tetlet.2008.01.060日期:2008.3S-Arylation of a wide variety of substituted aryl and aliphatic thiols with aryl halides catalyzed by copper iodide under mild ligand- and additive-free conditions (nBu4NBr, PhMe, NaOH, reflux, 22 h) is accomplished in good to excellent product yields (up to 96%).
表征谱图
-
氢谱1HNMR
-
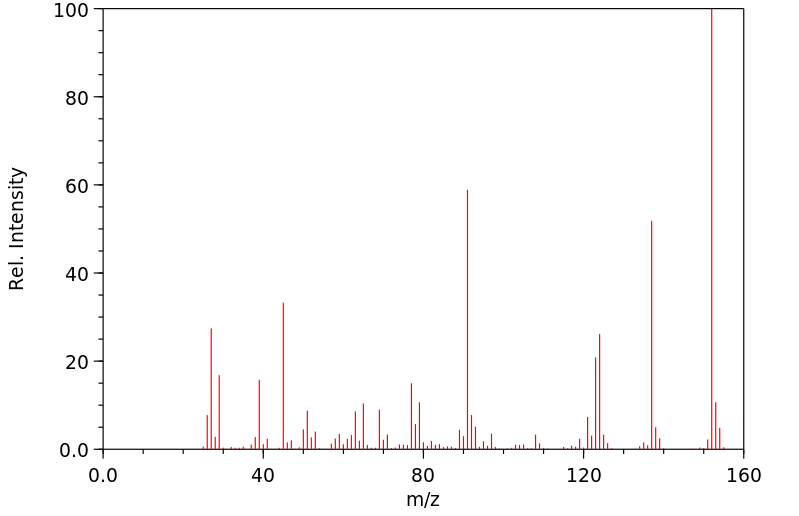
质谱MS
-
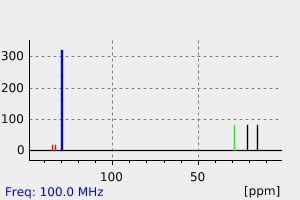
碳谱13CNMR
-
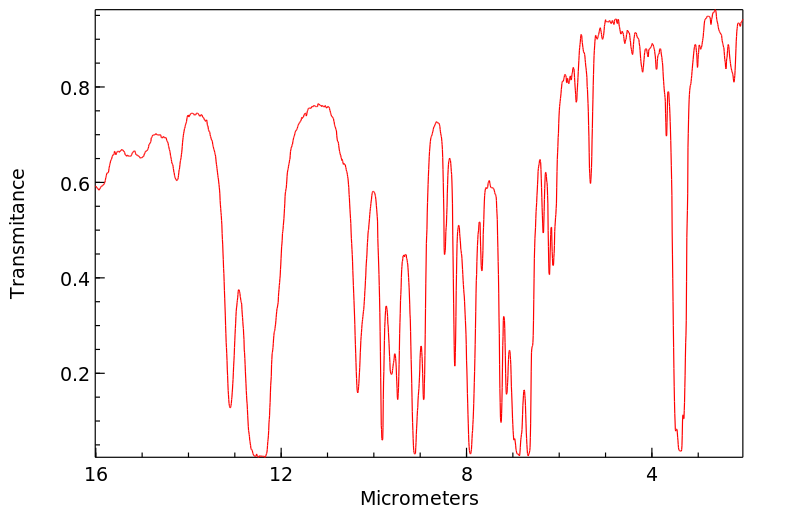
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯