2,5-二叔丁基硝基苯 | 3463-35-2
中文名称
2,5-二叔丁基硝基苯
中文别名
2,5-二-叔丁基硝基苯
英文名称
2,5-di-tert-butylnitrobenzene
英文别名
1,4-di-tert-butyl-2-nitrobenzene;1,4-di-tert-butyl-2-nitro-benzene;1,4-Di-tert-butyl-2-nitro-benzol;1,4-Di-tert.-butyl-2-nitrobenzol;1.4-Di-tert.-butyl-2-nitrobenzol;2-Nitro-1,4-di-tert-butyl-benzol;1,4-ditert-butyl-2-nitrobenzene
CAS
3463-35-2
化学式
C14H21NO2
mdl
MFCD00010337
分子量
235.326
InChiKey
LXBUSXRSPLOXCP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:88-90 °C(lit.)
-
沸点:303.0±21.0 °C(Predicted)
-
密度:1.002±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5.1
-
重原子数:17
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.571
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S26,S36
-
危险类别码:R20/21/22
-
海关编码:2904209090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2,5-di-tert-butyl-p-dinitrobenzene 10472-70-5 C14H20N2O4 280.324 —— 3-Nitro-2,5-di-t-butylanilin 22503-18-0 C14H22N2O2 250.341 —— 2,5-di-tert-butyl-1,3-dinitro-benzene 10472-73-8 C14H20N2O4 280.324 2,5-二叔丁基苯胺 2,5-di-tert-butylaniline 21860-03-7 C14H23N 205.343 —— 2,5-di-tert-butylbenzene-1,4-diamine 22162-01-2 C14H24N2 220.358 —— 2.5-di-tert-butyl-m-phenylenediamine 22503-14-6 C14H24N2 220.358 3,6-二(2-甲基-2-丙基)-1,2-苯二胺 3,6-di(tert-butyl)benzene-1,2-diamine 22503-12-4 C14H24N2 220.358
反应信息
-
作为反应物:描述:参考文献:名称:Burgers et al., Recueil des Travaux Chimiques des Pays-Bas, 1958, vol. 77, p. 491,506摘要:DOI:
-
作为产物:参考文献:名称:Alkylbenzenes from Benzene and Isobutene. I.1a Preparation, Identification of Several Fractions摘要:DOI:10.1021/ja01201a001
文献信息
-
Acylarylnitrosamines. Part II. The formation of arynes in the anomalous decompositions of o-t-butyl- and 2,5-di-t-butyl-N-nitrosoacetanilide作者:J. I. G. Cadogan、J. Cook、M. J. P. Harger、P. G. Hibbert、J. T. SharpDOI:10.1039/j29710000595日期:——3-t-butylbenzyne. 2,5-Di-t-butyl-N-nitrosoacetanilide similarly decomposes, in part, via 3,6-di-t-butylbenzyne. These abnormal decompositions are considered to arise as a result of preferential steric acceleration of unimolecular decomposition of the o-t-butylaryldiazonium cations to the corresponding carbonium ions, which either are converted into the ortho-acetates or to the aryne, which reacts with的分解ø -t -丁基- ñ -nitrosoacetanilide苯给出ø -和米-t-butylphenylacetates(55%)而不是2-叔丁基联苯的预期产量高。在呋喃,蒽和2,3,4,5-四苯基环戊二烯酮存在下的芳烃加合物的分离以及涉及蒽和9,10-二甲氧基蒽的芳烃竞争实验的结果确定了3-叔丁基苯并the的参与。2,5-二叔丁基-N-亚硝基乙酰苯胺类似地通过3,6-二叔丁基苯并de分解。这些异常分解被认为是由于o的单分子分解的优先空间加速而产生的-t-butylaryldiazonium阳离子为相应的碳离子,其或者被转换成邻-acetates或所述芳炔,用乙酸进行反应,得到米-乙酸甲酯。反过来,由于空间位阻,这些芳烃被认为反应性较低。
-
Nucleophilic Reactions of 5-<i>tert</i>-Butyl-2-methoxy-3<i>H</i>-azepine with Alkoxides and Alkyllithium Reagents作者:Yasuhiro Kubota、Kyosuke Satake、Ryusuke Ikui、Hideki Okamoto、Masaru KimuraDOI:10.1246/bcsj.76.805日期:2003.4The reaction of 5-tert-butyl-2-methoxy-3H-azepine (2a) with sodium alkoxides gave 2-alkoxy-3H-azepine derivatives 3–6 by nucleophilic transetherification. The treatment of 2a with tert-butyllithium also yielded 2,5-di-tert-butyl-3H-azepine (7); however, the reaction of 2a and methyllithium gave the expected 5-tert-butyl-2-methyl-3H-azepine (8) along with unexpected 5-tert-butyl-2,2-dimethyl-2,3-dihydro-1H-azepine5-叔丁基-2-甲氧基-3H-氮杂 (2a) 与醇钠反应通过亲核转醚反应得到 2-烷氧基-3H-氮杂衍生物 3-6。用叔丁基锂处理 2a 也产生了 2,5-二叔丁基-3H-氮杂 (7);然而,2a 和甲基锂的反应得到了预期的 5-叔丁基-2-甲基-3H-氮杂 (8) 以及意想不到的 5-叔丁基-2,2-二甲基-2,3-二氢-1H- azepine (9),还有 5,5'-di(tert-butyl)-2,2'-methylenedi(3H-azepine) (11),发现其结构是互变异构的 5-tert-butyl-2 -(5-叔丁基-2,3-二氢-1H-氮杂-2-亚基甲基)-3H-氮杂 (12)。基于 ab initio DFT 计算和动力学测量讨论了观察到的互变异构的能量分布。
-
Intramolecular Pd-Catalyzed Reductive Amination of Enolizable sp<sup>3</sup>-C–H Bonds作者:Russell L. Ford、Isabel Alt、Navendu Jana、Tom G. DriverDOI:10.1021/acs.orglett.9b03458日期:2019.11.1A palladium-catalyzed reductive cyclization of nitroarenes has been designed to construct sp3-C–NHAr bonds from sp3-C–H bonds by using an enolizable nucleophile to intercept a nitrosoarene intermediate. Exposure of ortho-substituted nitroarenes to 5 mol % of Pd(OAc)2 and 10 mol % of phenanthroline under 2 atm of CO constructs partially saturated 5-, 6-, or 7-membered N-heterocycles using α-pyridyl
-
Oximide derivatives and their therapeutical application申请人:Yu Chang Jun公开号:US20090012091A1公开(公告)日:2009-01-08The present invention relates to a compound represented as the following Formula (I) and a pharmaceutical composition thereof wherein all substituents are as defined in the specification; and also relates to a method for treating or lessening the severity of a disease or a condition, comprising administering said compound or said pharmaceutical composition.本发明涉及一种表示为以下化学式(I)的化合物及其药物组合物,其中所有取代基如规范中定义;还涉及一种治疗或减轻疾病或病况严重程度的方法,包括给予该化合物或该药物组合物。
-
Photoinduced Oxygen Transfer Using Nitroarenes for the Anaerobic Cleavage of Alkenes作者:Dan E. Wise、Emma S. Gogarnoiu、Alana D. Duke、Joshua M. Paolillo、Taylor L. Vacala、Waseem A. Hussain、Marvin ParasramDOI:10.1021/jacs.2c05648日期:2022.8.31Mechanistic studies support that the transformation occurs via direct photoexcitation of the nitroarene followed by a nonstereospecific radical cycloaddition event with alkenes. This leads to 1,3,2- and 1,4,2-dioxazolidine intermediates that fragment to give the carbonyl products. A combination of radical clock experiments and in situ photoNMR spectroscopy revealed the identities of the key radical species
表征谱图
-
氢谱1HNMR
-
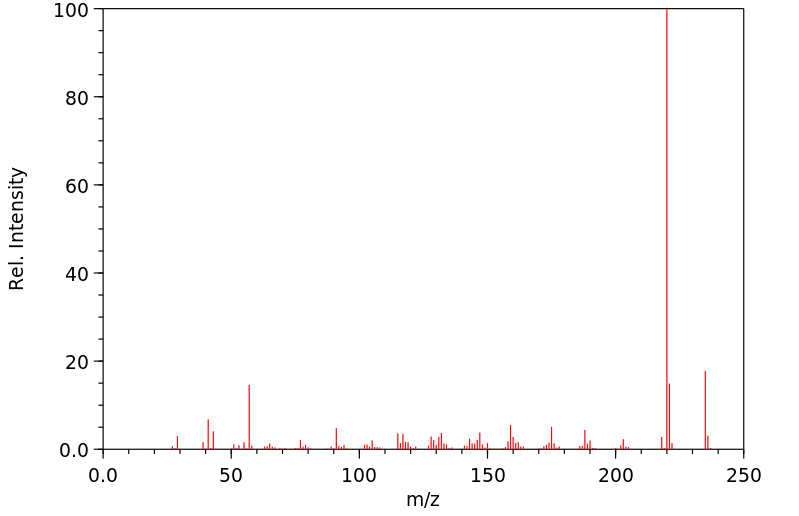
质谱MS
-
碳谱13CNMR
-
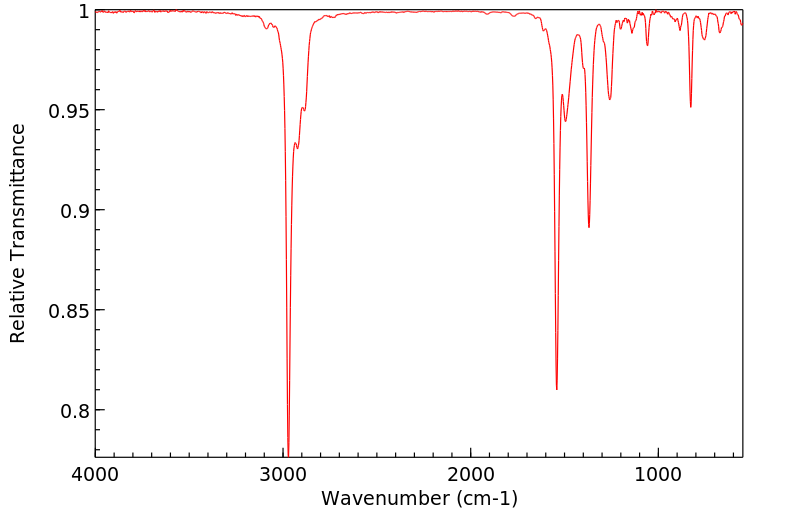
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫