furan-2-yl(morpholino)methanone | 31330-59-3
中文名称
——
中文别名
——
英文名称
furan-2-yl(morpholino)methanone
英文别名
2-furanyl 4-morpholinyl methanone;2-Furancarboxylic acid, morpholide;furan-2-yl(morpholin-4-yl)methanone
CAS
31330-59-3
化学式
C9H11NO3
mdl
MFCD00460930
分子量
181.191
InChiKey
MOZLMXOHIQAAND-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.444
-
拓扑面积:42.7
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:furan-2-yl(morpholino)methanone 在 正丁基锂 、 四氯化钛 作用下, 以 四氢呋喃 、 正己烷 、 二氯甲烷 为溶剂, 反应 34.5h, 生成 2,2-bis{5-[5-(α,α-dimethylfurfuryl)-α,α-dimethylfurfuryl]furan-2-yl}propane参考文献:名称:A rational approach to calix[n]furan precursors摘要:标题化合物的几种形式Cn可以通过线性前体的环化与丙酮和盐酸反应制得。已开发出一种两步法合成这些线性前体Ln(n = 4–9),从而可能合成标题化合物,包括:(i)从锂杂呋喃和三级酰胺合成相应的单或双酮;(ii)使用四氯化钛和二甲基锌对酮基进行顺二甲基化。DOI:10.1039/b108402j
-
作为产物:描述:参考文献:名称:活化仲酰胺的无催化剂,无金属和化学选择性转氨基摘要:摘要 据报道,在温和条件下,无溶剂,无金属和化学选择性的简单方案可用于乙醇中作为溶剂的活化仲酰胺的氨基转移。在该方法学中容许在催化的转酰胺基化中有问题的各种各样的胺,氨基酸,氨基醇和取代基。转酰胺基反应也成功地扩展到水作为介质。本方法学似乎比最近报道的其他催化的转酰胺作用更好。 据报道,在温和条件下,无溶剂,无金属和化学选择性的简单方案可用于乙醇中作为溶剂的活化仲酰胺的氨基转移。在该方法学中容许在催化的转酰胺基化中有问题的各种各样的胺,氨基酸,氨基醇和取代基。转酰胺基反应也成功地扩展到水作为介质。本方法学似乎比最近报道的其他催化的转酰胺作用更好。DOI:10.1055/s-0037-1610664
文献信息
-
Dehydrogenative amide synthesis from alcohol and amine catalyzed by hydrotalcite-supported gold nanoparticles作者:Jiangling Zhu、Yan Zhang、Feng Shi、Youquan DengDOI:10.1016/j.tetlet.2012.04.048日期:2012.6Hydrotalcite-supported nano-gold (Au/HT) was found to be a highly efficient heterogeneous catalyst for the dehydrogenative synthesis of amide from alcohol and amine. Amines and alcohols with different structures could be converted into the amides under mild reaction conditions with up to 98% isolated yields. Mechanism exploration suggested that ester might be the reaction intermediate.
-
Superparamagnetic Fe(OH)<sub>3</sub>@Fe<sub>3</sub>O<sub>4</sub> Nanoparticles: An Efficient and Recoverable Catalyst for Tandem Oxidative Amidation of Alcohols with Amine Hydrochloride Salts作者:Marzban Arefi、Dariush Saberi、Meghdad Karimi、Akbar HeydariDOI:10.1021/co5001844日期:2015.6.8nanoparticles were successfully prepared and characterized. This magnetic nanocomposite was employed as an efficient, reusable, and environmentally benign heterogeneous catalyst for the direct amidation of alcohols with amine hydrochloride salts. Several derivatives of primary, secondary and tertiary amides were synthesized in moderate to good yields in the presence of this catalytic system. The catalyst was磁性Fe(OH)3 @Fe 3 O 4纳米粒子的制备和表征成功。该磁性纳米复合材料用作将醇与胺盐酸盐直接酰胺化的有效,可重复使用且对环境无害的多相催化剂。在该催化体系的存在下,以中等至良好的产率合成了伯,仲和叔酰胺的几种衍生物。该催化剂已成功回收利用,最多可重复使用六次,而不会显着降低其催化活性。
-
Manganese Catalyzed Direct Amidation of Esters with Amines作者:Zhengqiang Fu、Xinghua Wang、Sheng Tao、Qingqing Bu、Donghui Wei、Ning LiuDOI:10.1021/acs.joc.0c02478日期:2021.2.5metal catalyzed amidations remains a challenge. Here, a manganese(I)-catalyzed method for the direct synthesis of amides from a various number of esters and amines is reported with unprecedented substrate scope using a low catalyst loading. A wide range of aromatic, aliphatic, and heterocyclic esters, even in fatty acid esters, reacted with a diverse range of primary aryl amines, primary alkyl amines
-
从羧酸以铱、钴配合物为催化剂并在蓝光照射下制备酰胺的方法申请人:大连理工大学公开号:CN112778238B公开(公告)日:2022-10-28
-
Phenysilane and Silicon Tetraacetate: Versatile Promotors for Amide Synthesis作者:Eléonore Morisset、Aurélien Chardon、Jacques Rouden、Jérôme BlanchetDOI:10.1002/ejoc.201901660日期:2020.1.23Phenylsilane as been reassed as useful coupling reagent for amides synthesis. Peptides and Weinreb amides are easily obtained under mild conditions in absence of epimzerization even with challenging substrates such as phenylglycine. Further investigation regarding the reactivity of stable acetoxysilanes supported phenylpolyacyloxysilane as key intermediate.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
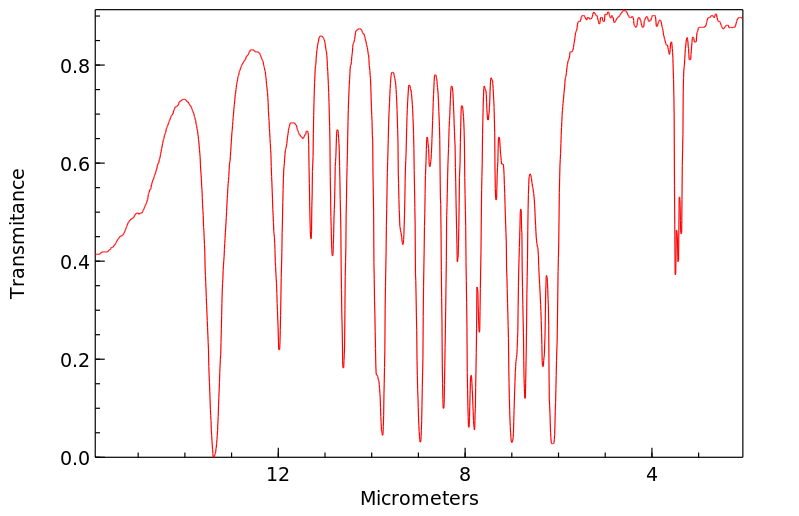
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(4-甲基环戊-1-烯-1-基)(吗啉-4-基)甲酮
(2-肟基-氰基乙酸乙酯)-N,N-二甲基-吗啉基脲六氟磷酸酯
鲸蜡基乙基吗啉氮鎓乙基硫酸盐
马啉乙磺酸钾
预分散OTOS-80
顺式4-(氮杂环丁烷-3-基)-2,2-二甲基吗啉
顺式-N-亚硝基-2,6-二甲基吗啉
顺式-3,5-二甲基吗啉
顺-2,6-二甲基-4-(4-硝基苯基)吗啉
非屈酯
雷奈佐利二聚体
阿瑞杂质9
阿瑞杂质12
阿瑞吡坦磷的二卞酯
阿瑞吡坦杂质
阿瑞吡坦杂质
阿瑞吡坦EP杂质C
阿瑞吡坦
阿瑞吡坦
阿瑞匹坦非对映异构体2R3R1R
阿瑞匹坦杂质A异构体
阿瑞匹坦杂质54
阿瑞匹坦-M3代谢物
钾[2 - (吗啉- 4 -基)乙氧基]甲基三氟硼酸
酮康唑杂质
邻苯二甲酸单吗啉
调节安
试剂2-(4-Morpholino)ethyl2-bromoisobutyrate
茂硫磷
苯甲腈,2-(4-吗啉基)-5-[1,4,5,6-四氢-4-(羟甲基)-6-羰基-3-哒嗪基]-
苯甲曲秦
苯甲吗啉酮
苯基2-(2-苯基吗啉-4-基)乙基碳酸酯盐酸盐
苯二甲吗啉一氢酒石酸盐
苯二甲吗啉
苯乙酮 O-(吗啉基羰基甲基)肟
芬美曲秦
芬布酯盐酸盐
芬布酯
脾脏酪氨酸激酶(SYK)抑制剂
脱氯利伐沙班
脱氟雷奈佐利
羟基1-(3-氯苯基)-2-[(1,1-二甲基乙基)氨基]-1-丙酮盐酸盐
福沙匹坦苄酯
福沙匹坦杂质26
福沙匹坦N-苄基杂质
福曲他明
碘化N-甲基丙基吗啉
碘化N-甲基,乙基吗啉
硝酸吗啉