苯胺氢溴酸盐 | 542-11-0
中文名称
苯胺氢溴酸盐
中文别名
——
英文名称
aniline hydrobromide
英文别名
anilinium bromide;phenylazanium;bromide
CAS
542-11-0
化学式
Br*C6H8N
mdl
——
分子量
174.04
InChiKey
KBPWECBBZZNAIE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:285°C(dec.)(lit.)
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
计算性质
-
辛醇/水分配系数(LogP):1.85
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:26
-
氢给体数:2
-
氢受体数:1
安全信息
-
海关编码:2921419000
-
WGK Germany:3
-
储存条件:本品应密封避光保存。
SDS
Version 2.1
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name ANILINE HYDROBROMIDE - 50 MG
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
Toxic by inhalation, in contact with skin and if swallowed.
Limited evidence of a carcinogenic effect. Risk of serious damage
to eyes. May cause sensitization by skin contact. Toxic: danger of
serious damage to health by prolonged exposure through inhalation,
in contact with skin and if swallowed. Possible risk of
irreversible effects. Very toxic to aquatic organisms.
Carc. Cat.3 Muta. Cat.3
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
ANILINE HYDROBROMIDE 542-11-0 208-801-9 612-009-00-2
Formula C6H7N
Molecular Weight 174,0400 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
ALDRICH www.molbase.com
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician immediately.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
appropriate foam.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PERSONAL PRECAUTION PROCEDURES TO BE FOLLOWED IN CASE OF LEAK OR SPILL
Evacuate area.
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear self-contained breathing apparatus, rubber boots, and heavy
rubber gloves.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Do not breathe dust. Do not get in
eyes, on skin, on clothing. Avoid prolonged or repeated exposure.
STORAGE
Conditions of Storage: Keep tightly closed.
SPECIAL REQUIREMENTS: Light sensitive.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Use only in a chemical fume hood. Safety shower and eye bath.
GENERAL HYGIENE MEASURES
Wash contaminated clothing before reuse. Wash thoroughly after
handling.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Where risk assessment shows air-purifying respirators
are appropriate use a full-face particle respirator type N99 (US)
or type P2 (EN 143) respirator cartridges as a backup to
engineering controls. If the respirator is the sole means of
protection, use a full-face supplied air respirator.
Hand Protection: Compatible chemical-resistant gloves.
ALDRICH www.molbase.com
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
Appearance Physical State: Solid
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Nitrogen oxides, Hydrogen bromide gas.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
SENSITIZATION
Sensitization: Sensitizer.
Skin: May cause allergic skin reaction.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: Toxic if absorbed through skin.
Eye Contact: Causes severe eye irritation.
Inhalation: Material may be irritating to mucous membranes and
upper respiratory tract. Toxic if inhaled.
Ingestion: Toxic if swallowed.
CHRONIC EXPOSURE - CARCINOGEN
ALDRICH www.molbase.com
Result: This product is or contains a component that has been
reported to be possibly carcinogenic based on its IARC, ACGIH,
NTP, or EPA classification.
CHRONIC EXPOSURE - MUTAGEN
Result: May alter genetic material.
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. Dissolve or mix the material with a combustible
solvent and burn in a chemical incinerator equipped with an
afterburner and scrubber. Observe all federal, state, and local
environmental regulations.
14 - Transport Information
RID/ADR
UN#: 2811
Class: 6.1
PG: III
Proper Shipping Name: Toxic solid, organic, n.o.s.
IMDG
UN#: 2811
Class: 6.1
PG: III
Proper Shipping Name: Toxic solid, organic, n.o.s.
Marine Pollutant: No
Severe Marine Pollutant: No
Technical Name: Required
IATA
UN#: 2811
Class: 6.1
PG: III
Proper Shipping Name: Toxic solid, organic, n.o.s.
Inhalation Packing Group I: No
Technical Name: Required
15 - Regulatory Information
CLASSIFICATION AND LABELING ACCORDING TO EU DIRECTIVES
ANNEX I INDEX NUMBER: 612-009-00-2
NOTA: A
INDICATION OF DANGER: T-N
Toxic. Dangerous for the environment.
R-PHRASES: 23/24/25-40-41-43-48/23/24/25-68-50
Toxic by inhalation, in contact with skin and if swallowed.
Limited evidence of a carcinogenic effect. Risk of serious
damage to eyes. May cause sensitization by skin contact. Toxic:
danger of serious damage to health by prolonged exposure
through inhalation, in contact with skin and if swallowed.
Possible risk of irreversible effects. Very toxic to aquatic
organisms.
ALDRICH www.molbase.com
S-PHRASES: 26-27-36/37/39-45-61-63
In case of contact with eyes, rinse immediately with plenty of
water and seek medical advice. Take off immediately all
contaminated clothing. Wear suitable protective clothing,
gloves, and eye/face protection. In case of accident or if you
feel unwell, seek medical advice immediately (show the label
where possible). Avoid release to the environment. Refer to
special instructions/safety data sheets. In case of accident by
inhalation: remove casualty to fresh air and keep at rest.
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name ANILINE HYDROBROMIDE - 50 MG
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
Toxic by inhalation, in contact with skin and if swallowed.
Limited evidence of a carcinogenic effect. Risk of serious damage
to eyes. May cause sensitization by skin contact. Toxic: danger of
serious damage to health by prolonged exposure through inhalation,
in contact with skin and if swallowed. Possible risk of
irreversible effects. Very toxic to aquatic organisms.
Carc. Cat.3 Muta. Cat.3
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
ANILINE HYDROBROMIDE 542-11-0 208-801-9 612-009-00-2
Formula C6H7N
Molecular Weight 174,0400 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
ALDRICH www.molbase.com
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician immediately.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
appropriate foam.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PERSONAL PRECAUTION PROCEDURES TO BE FOLLOWED IN CASE OF LEAK OR SPILL
Evacuate area.
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear self-contained breathing apparatus, rubber boots, and heavy
rubber gloves.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Do not breathe dust. Do not get in
eyes, on skin, on clothing. Avoid prolonged or repeated exposure.
STORAGE
Conditions of Storage: Keep tightly closed.
SPECIAL REQUIREMENTS: Light sensitive.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Use only in a chemical fume hood. Safety shower and eye bath.
GENERAL HYGIENE MEASURES
Wash contaminated clothing before reuse. Wash thoroughly after
handling.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Where risk assessment shows air-purifying respirators
are appropriate use a full-face particle respirator type N99 (US)
or type P2 (EN 143) respirator cartridges as a backup to
engineering controls. If the respirator is the sole means of
protection, use a full-face supplied air respirator.
Hand Protection: Compatible chemical-resistant gloves.
ALDRICH www.molbase.com
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
Appearance Physical State: Solid
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Nitrogen oxides, Hydrogen bromide gas.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
SENSITIZATION
Sensitization: Sensitizer.
Skin: May cause allergic skin reaction.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: Toxic if absorbed through skin.
Eye Contact: Causes severe eye irritation.
Inhalation: Material may be irritating to mucous membranes and
upper respiratory tract. Toxic if inhaled.
Ingestion: Toxic if swallowed.
CHRONIC EXPOSURE - CARCINOGEN
ALDRICH www.molbase.com
Result: This product is or contains a component that has been
reported to be possibly carcinogenic based on its IARC, ACGIH,
NTP, or EPA classification.
CHRONIC EXPOSURE - MUTAGEN
Result: May alter genetic material.
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. Dissolve or mix the material with a combustible
solvent and burn in a chemical incinerator equipped with an
afterburner and scrubber. Observe all federal, state, and local
environmental regulations.
14 - Transport Information
RID/ADR
UN#: 2811
Class: 6.1
PG: III
Proper Shipping Name: Toxic solid, organic, n.o.s.
IMDG
UN#: 2811
Class: 6.1
PG: III
Proper Shipping Name: Toxic solid, organic, n.o.s.
Marine Pollutant: No
Severe Marine Pollutant: No
Technical Name: Required
IATA
UN#: 2811
Class: 6.1
PG: III
Proper Shipping Name: Toxic solid, organic, n.o.s.
Inhalation Packing Group I: No
Technical Name: Required
15 - Regulatory Information
CLASSIFICATION AND LABELING ACCORDING TO EU DIRECTIVES
ANNEX I INDEX NUMBER: 612-009-00-2
NOTA: A
INDICATION OF DANGER: T-N
Toxic. Dangerous for the environment.
R-PHRASES: 23/24/25-40-41-43-48/23/24/25-68-50
Toxic by inhalation, in contact with skin and if swallowed.
Limited evidence of a carcinogenic effect. Risk of serious
damage to eyes. May cause sensitization by skin contact. Toxic:
danger of serious damage to health by prolonged exposure
through inhalation, in contact with skin and if swallowed.
Possible risk of irreversible effects. Very toxic to aquatic
organisms.
ALDRICH www.molbase.com
S-PHRASES: 26-27-36/37/39-45-61-63
In case of contact with eyes, rinse immediately with plenty of
water and seek medical advice. Take off immediately all
contaminated clothing. Wear suitable protective clothing,
gloves, and eye/face protection. In case of accident or if you
feel unwell, seek medical advice immediately (show the label
where possible). Avoid release to the environment. Refer to
special instructions/safety data sheets. In case of accident by
inhalation: remove casualty to fresh air and keep at rest.
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法: 有机合成。
用途简介: 暂无内容。
用途: 有机合成。
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis of Deuterated Biphenyls1摘要:DOI:10.1021/jo01089a014
-
作为产物:参考文献:名称:通过 BBr3 介导的叠氮化物串联还原和甲醚脱保护直接合成氨基-3-羟基吡啶-4-one 铁螯合剂摘要:摘要 描述了一种新颖且直接的 BBr3 介导的有机叠氮化物串联还原和甲基醚脱甲基作用。该方法为制备一系列新型氨基-3-羟基吡啶-4-one铁螯合剂提供了一种新途径,该螯合剂可用于铁的处理,也适用于苄基叠氮化物和芳基叠氮化物等其他底物。图形概要DOI:10.1080/00397911.2018.1542002
-
作为试剂:描述:参考文献:名称:Reddelien, Chemische Berichte, 1915, vol. 48, p. 1468摘要:DOI:
文献信息
-
Selective catalytic Hofmann N-alkylation of poor nucleophilic amines and amides with catalytic amounts of alkyl halides作者:Qing Xu、Huamei Xie、Er-Lei Zhang、Xiantao Ma、Jianhui Chen、Xiao-Chun Yu、Huan LiDOI:10.1039/c6gc00938g日期:——A selective Hofmann N-alkylation reaction of amines/amides catalytic in alkyl halides is achieved by using alcohols as the alkylating reagents, affording mono- or di-alkylated amines/amides in high selectivities.通过使用醇作为烷基化试剂,实现烷基卤化物中催化的胺/酰胺的霍夫曼N-烷基的选择性霍夫曼N-烷基化反应,从而以高选择性提供单或二烷基化的胺/酰胺。
-
MEMS for Light-Wave Networks作者:C. Randy Giles、David Bishop、Vladimir AksyukDOI:10.1557/mrs2001.73日期:2001.4
As demonstrated in this issue, the emerging field of microelectromechanical systems (MEMS) is beginning to impact almost every area of science and technology. MEMS have the potential to revolutionize light-wave systems. Microdevices such as optical switches, variable attenuators, active equalizers, add/drop multiplexers (ADMs), optical cross-connects (OXCs), gain tilt equalizers, data transmitters, and many others are beginning to find ubiquitous application in advanced light-wave systems.
-
Selective N-alkylation of primary amines with R–NH2·HBr and alkyl bromides using a competitive deprotonation/protonation strategy作者:Shubhankar Bhattacharyya、Uma Pathak、Sweta Mathur、Subodh Vishnoi、Rajeev JainDOI:10.1039/c4ra01915f日期:——Monoalkylation of primary amines using amine hydrobromides and alkyl bromides has been carried out. Under controlled reaction conditions the reactant primary amine was selectively deprotonated and made available for reaction, while the newly generated secondary amine remained protonated, and did not participate in alkylation further. Reaction was carried out under mild reaction conditions and was applicable to a wide range of primary amines and alkyl bromides.使用胺溴化物和卤代烷对初级胺进行单烷基化已成功实现。在受控反应条件下,反应物初级胺被选择性去质子化,从而可供反应,而新生成的次级胺保持质子化状态,未进一步参与烷基化反应。反应在温和的条件下进行,并适用于多种初级胺和卤代烷。
-
Thiopegan derivatives—XXI作者:G.M. Sharma、H.S. Sachdev、N.K. Ralhan、H. Singh、G. Sarjit Sandhu、K. Gandhi、K.S. NarangDOI:10.1016/0040-4020(61)80007-0日期:1961.1Reactions between α-haloketones and o-carbethoxy phenyl thiourea have been discussed; a mechanism for the observed exclusive formation of 9:10-thiopega-2:10-diene-4-ones in the case of α-haloalkaryl ketones has been advanced and a number of intermediates isolated in case of chloro acetone.
-
Development of quinone analogues as dynamin GTPase inhibitors作者:Kylie A. MacGregor、Mohammed K. Abdel-Hamid、Luke R. Odell、Ngoc Chau、Ainslie Whiting、Phillip J. Robinson、Adam McCluskeyDOI:10.1016/j.ejmech.2014.06.070日期:2014.10Virtual screening of the ChemDiversity and ChemBridge compound databases against dynamin I (dynI) GTPase activity identified 2,5-bis-(benzylamino)-1,4-benzoquinone 1 as a 273 ± 106 μM inhibitor. In silico lead optimization and focused library-led synthesis resulted in the development of four discrete benzoquinone/naphthoquinone based compound libraries comprising 54 compounds in total. Sixteen analogues针对dynamin I(dynI)GTPase活性对ChemDiversity和ChemBridge化合物数据库进行的虚拟筛选确定了2,5-双-(苄氨基)-1,4-苯醌1为273±106μM抑制剂。在计算机上进行了铅优化和以库为主导的合成,开发了四个基于苯醌/萘醌的化合物库,总共包含54种化合物。16种类似物比铅1更有效,其中2,5-双-(4-羟基苯胺基)-1,4-苯醌(45)和2,5-双(4-羧基苯胺基)-1,4-苯醌(49) IC 50最活跃值分别为11.1±3.6和10.6±1.6μM。分子建模表明,dynI GTP结合位点内49个分子的稳定化涉及许多氢键和疏水相互作用。评价了六个活性最高的抑制剂对网格蛋白介导的内吞作用(CME)的潜在抑制作用。醌45是最有效的CME抑制剂,IC 50(CME)为36±16μM。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
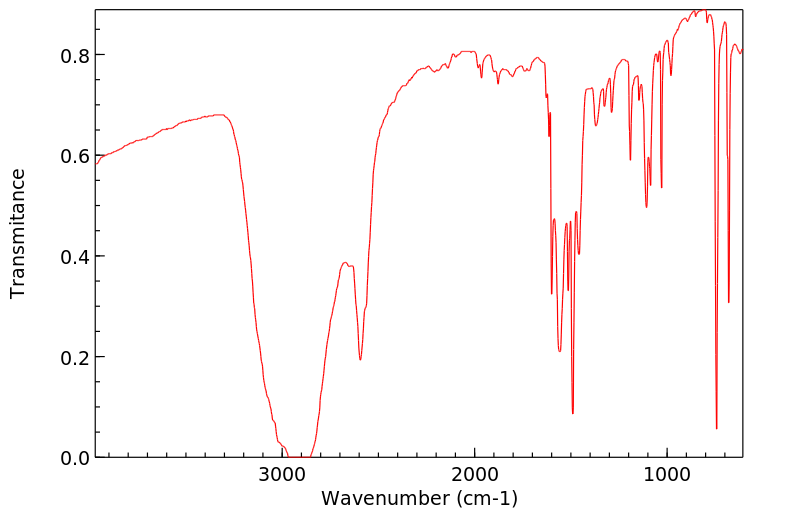
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫