acetic acid-[N'-(2,4-dinitro-phenyl)-hydrazide] | 2719-07-5
中文名称
——
中文别名
——
英文名称
acetic acid-[N'-(2,4-dinitro-phenyl)-hydrazide]
英文别名
N-(2,4-dinitrophenyl)-N'-acetylhydrazine;acetic acid 2,4-dinitrophenylhydrazide;N'-(2,4-dinitrophenyl)acetohydrazide;acetic 2,4-dinitrophenylhydrazide;2,4-dinitrophenylacetylhydrazine;Essigsaeure-[N'-(2,4-dinitro-phenyl)-hydrazid]
CAS
2719-07-5
化学式
C8H8N4O5
mdl
——
分子量
240.175
InChiKey
NYZGFEVPHFYXKA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:17
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:133
-
氢给体数:2
-
氢受体数:6
上下游信息
反应信息
-
作为反应物:描述:acetic acid-[N'-(2,4-dinitro-phenyl)-hydrazide] 在 甲醇 、 ammonium cerium (IV) nitrate 作用下, 生成 1,3-二硝基苯参考文献:名称:CAN-Mediated Oxidation of Electron-Deficient Aryl and Heteroaryl Hydrazines and Hydrazides摘要:芳基和杂芳基肼及肼酰胺成功地通过CAN被氧化,生成去肼化产物。反应途径强烈依赖于底物的性质,导致烃或烷氧衍生物的形成。当使用氘化溶剂,如氘代甲醇(methanol-d₄)或氘代乙腈(acetonitrile-d₃)时,实现了氘的区域特异性结合。DOI:10.1055/s-2007-1072749
-
作为产物:描述:参考文献:名称:Curtius; Mayer, Journal fur praktische Chemie (Leipzig 1954), 1907, vol. <2> 76, p. 380摘要:DOI:
文献信息
-
Metal Acetate/Metal Oxide in Acetic Acid: An Efficient Reagent for the Chemoselective N-Acetylation of Amines under Green Conditions作者:Goutam Brahmachari、Sujay Laskar、Sajal SarkarDOI:10.3184/030823410x12746305905926日期:2010.5The use of catalytic amount of metal acetate or metal oxide in acetic acid is a new and highly efficient acetylating system for chemoselective N-acetylation of primary and secondary amines in excellent yields under reflux condition. No other solvent is required. The noted features of this method include mild reaction conditions, use of innocuous reagents, excellent yields, convenient work-up, and reuse
-
An Approach to Pyrazoline-Fused Chlorins by Dipolar [3 + 2]-Cycloaddition of Iminonitriles to <i>meso</i>-Tetrakis(pentafluorophenyl)porphyrin: Synthesis of New PDT Photosensitizers作者:Przemysaw Wyrebek、Stanisaw OstrowskiDOI:10.1246/bcsj.20110408日期:2012.10.15meso-Tetrakis(pentafluorophenyl)porphyrin reacts at higher temperature with unstable iminonitriles (R–C≡N+–N−–Ar), affording pyrazoline-fused chlorins, according to dipolar [3 + 2]-cycloaddition pathway. The respective iminonitriles were in situ generated from the corresponding functionalized α-halogenohydrazine derivatives by 1,3-elimination of HX in the presence of base (NEt3, DABCO). This method allows synthesis of very attractive moieties which may be of potential use as sensitizers in photodynamic therapy (PDT).
-
Simultaneous Determination of C<sub>1</sub>−C<sub>4</sub> Carboxylic Acids and Aldehydes Using 2,4-Dinitrophenylhydrazine-Impregnated Silica Gel and High-Performance Liquid Chromatography作者:Shigehisa Uchiyama、Erika Matsushima、Shohei Aoyagi、Masanori AndoDOI:10.1021/ac0493471日期:2004.10.14-dinitrophenylhydrazides exhibited maximum absorption wavelengths of 331-334 nm and molar absorption coefficients of 1.4 x 10(4) L/mol/cm. They were completely separated by high-performance liquid chromatography (HPLC) with an RP-Amide C16 column. Cartridges packed with DNPH-coated silica particles (DNPH cartridge) were used for sampling formic acid and aldehydes. Formic acid was physically adsorbed on描述了一种同时测定空气中脂肪族羧酸和醛类的新方法。在这项工作中,使羧酸与2,4-二硝基苯肼(DNPH)反应形成相应的羧基2,4-二硝基苯肼。这些衍生物具有出色的热稳定性,其熔点比相应的高32-50摄氏度。C1-C4羧酸2,4-二硝基苯酰肼的最大吸收波长为331-334 nm,摩尔吸收系数为1.4 x 10(4)L / mol / cm。使用RP-Amide C16色谱柱的高效液相色谱(HPLC)将它们完全分离。使用装有DNPH涂层的二氧化硅颗粒的小柱(DNPH小柱)对甲酸和醛进行采样。作为采样机制的第一步,甲酸物理吸附在二氧化硅颗粒上。随后与DNPH逐渐反应。甲酸在室温(20摄氏度)下与DNPH反应非常缓慢,但在80摄氏度下历时4小时完全反应。在现场测量中,通过DNPH滤芯吸入样品空气。取样后,将小柱在80摄氏度下加热5小时,然后用乙腈萃取进行HPLC分析。在这些优化条件下,对于以100 mL
-
Purgotti, Gazzetta Chimica Italiana, 1894, vol. 24 I, p. 568作者:PurgottiDOI:——日期:——
-
Brahmachari; Laskar; Sarkar, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2010, vol. 49, # 9, p. 1274 - 1281作者:Brahmachari、Laskar、SarkarDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
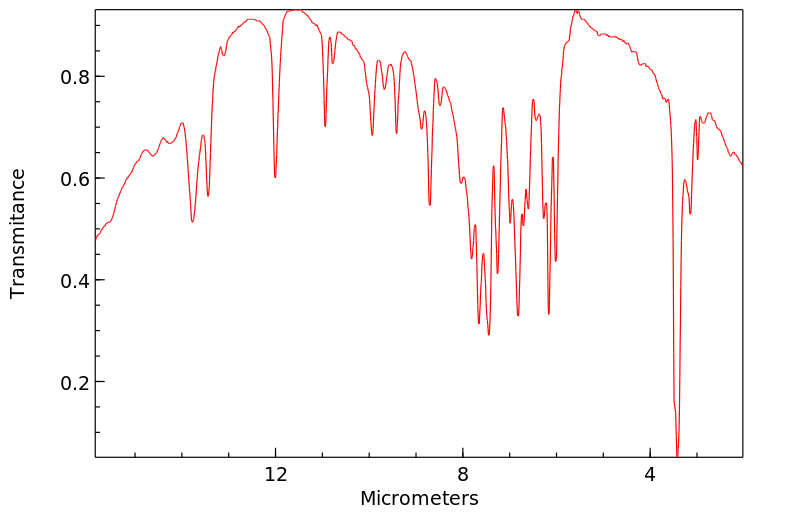
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫