苯磺酸2-氯乙酯 | 16670-48-7
中文名称
苯磺酸2-氯乙酯
中文别名
——
英文名称
2-chloroethyl benzenesulfonate
英文别名
2-(benzene-sulphonyloxy)ethyl chloride;benzenesulfonic acid-(2-chloro-ethyl ester);Benzolsulfonsaeure-(2-chlor-aethylester)
CAS
16670-48-7
化学式
C8H9ClO3S
mdl
MFCD10566886
分子量
220.677
InChiKey
CIIGWOXXOUVEAD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:188 °C / 17mmHg
-
密度:1.35
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:51.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2905590090
-
储存条件:存放于惰性气体中,并避免接触湿气(否则可能分解)。
SDS
2-Chloroethyl Benzenesulfonate Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2-Chloroethyl Benzenesulfonate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2-Chloroethyl Benzenesulfonate
Percent: >98.0%(GC)
CAS Number: 16670-48-7
Synonyms: Benzenesulfonic Acid 2-Chloroethyl Ester
Chemical Formula: C8H9ClO3S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
2-Chloroethyl Benzenesulfonate
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Very pale yellow - Pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
188°C/2.3kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
1.35
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
2-Chloroethyl Benzenesulfonate
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2-Chloroethyl Benzenesulfonate
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2-Chloroethyl Benzenesulfonate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2-Chloroethyl Benzenesulfonate
Percent: >98.0%(GC)
CAS Number: 16670-48-7
Synonyms: Benzenesulfonic Acid 2-Chloroethyl Ester
Chemical Formula: C8H9ClO3S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
2-Chloroethyl Benzenesulfonate
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Very pale yellow - Pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
188°C/2.3kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
1.35
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
2-Chloroethyl Benzenesulfonate
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2-Chloroethyl Benzenesulfonate
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:烷基和取代烷基甲磺酸盐的制备和一些裂解反应:氟化物、碘化物和硫氰酸盐的合成摘要:合成了具有代表性的甲磺酸酯,并裂解形成相应的氟化物、碘化物和硫氰酸酯。从这项工作中开发了一种方便的实验室程序,用于将醇转化为氟化物。DOI:10.1139/v56-099
-
作为产物:描述:参考文献:名称:Mansurow; Zukerwanik, Doklady Akademii Nauk UzSSR, 1957, # 12, p. 23,24摘要:DOI:
文献信息
-
Reaction of Pyrylium Salts with Nucleophiles. 23: Triarylethene Derivatives Containing an Oxyalkyleneamino or Oxyalkylene-N-pyridinium Side Chain作者:Octavian Stanciuc、Alexandru T. BalabanDOI:10.1002/jps.2600820911日期:1993.9amines related to anticancer drugs clomiphene and tamoxifen on the basis of key intermediates with a phenolic group, to which a side chain (omega-aminoethoxy or omega-aminopropoxy) was attached. These compounds were then reacted with 2,4,6-trimethyl- or 2,4,6-triphenylpyrylium salts. This afforded pyridinium analogues of clomiphene and tamoxifen as potential therapeutic agents for treatment against hormone-dependent
-
Anti-arrhythmic agents
-
PDE9 inhibitors for treating cardiovascular disorders申请人:Pfizer Inc.公开号:US20030195205A1公开(公告)日:2003-10-16The invention relates to PDE9 inhibitors for treating cardiovascular disorders. Preferred PDE9 inhibitors are compounds of formula I wherein R 1 is H or C 1-6 alkyl, wherein R 1 is attached to either N 1 or N 2 ; R 2 is C 1-6 alkyl optionally substituted by hydroxy or alkoxy; C 3-7 cycloalkyl optionally substituted by alkyl, hydroxy or alkoxy; a saturated 5-6-membered heterocycle optionally substituted by alkyl, hydroxy or alkoxy; het1 or Ar 1 ; R 3 is C 1-6 alkyl optionally substituted by 1 or 2 groups independently selected from: Ar 2 ; C 3-7 cycloalkyl optionally substituted by C 1-6 alkyl; OAr 2 ; SAr 2 ; NHC(O)C 1-6 alkyl; het 2 ; xanthene; and naphthalene. 1
-
Heterocyclic thromboxane synthetase inhibitors and pharmaceutical申请人:Pfizer Inc.公开号:US04410539A1公开(公告)日:1983-10-18Heterocyclic thromboxane synthetase inhibitors of the formula ##STR1## wherein R.sup.1, which is attached to the 2-, 3- or 4-position, is hydrogen, halogen, C.sub.1 -C.sub.4 alkyl, hydroxy or C.sub.1 -C.sub.4 alkoxy; Y, which is attached to the 2- or 3-position, is --COOH, --COO(C.sub.1 -C.sub.4 alkyl) or --CONH.sub.2 ; X is O, S, NH, N(C.sub.1 -C.sub.4 alkyl) or N(benzyl); and R, which is attached to the 5-, 6- or 7-position, is a group of the formula ##STR2## or (3- or 4-pyridyl)-Z.sup.2 - wherein Z.sup.1 is --CH.sub.2 --, --CH.sub.2 CH.sub.2 -- or --CH.sub.2 CH.sub.2 O-- and Z.sup.2 is --CH.sub.2 --, --CH.sub.2 CH.sub.2 --, --CH.dbd.CH--, --CH.sub.2 O-- or --OCH.sub.2 --; and their pharmaceutically acceptable salts; processes for their preparation, and pharmaceutical compositions containing them.杂环血栓素合成酶抑制剂的化学式为##STR1##其中R.sup.1,连接到2-、3-或4-位置,是氢、卤素、C.sub.1 -C.sub.4烷基、羟基或C.sub.1 -C.sub.4烷氧基;Y,连接到2-或3-位置,是--COOH、--COO(C.sub.1 -C.sub.4烷基)或--CONH.sub.2;X为O、S、NH、N(C.sub.1 -C.sub.4烷基)或N(苄基);R,连接到5-、6-或7-位置,是化学式##STR2##或(3-或4-吡啶基)-Z.sup.2-其中Z.sup.1为--CH.sub.2 --、--CH.sub.2 CH.sub.2 --或--CH.sub.2 CH.sub.2 O--,Z.sup.2为--CH.sub.2 --、--CH.sub.2 CH.sub.2 --、--CH.dbd.CH--、--CH.sub.2 O--或--OCH.sub.2 --;以及它们的药学上可接受的盐;它们的制备方法和含有它们的药物组合物。
-
Antiarrhythmic agents, compositions and method of use thereas申请人:Pfizer Inc.公开号:US04891372A1公开(公告)日:1990-01-02An antiarrhythmic agent of the formula ##STR1## or a pharmaceutically acceptable salt thereof; wherein R.sup.1 is H, C.sub.1 -C.sub.4 alkyl or C.sub.1 -C.sub.4 alkoxy; X is O, ##STR2## or a direct link; and R.sup.2 and R.sup.3, which are the same or different, are each C.sub.1 -C.sub.4 alkyl, with the proviso that when X is ##STR3## R.sup.2 and R.sup.3 are the same.一种抗心律失常药剂,其分子式为##STR1##或其药用可接受的盐;其中R.sup.1为H、C.sub.1 -C.sub.4烷基或C.sub.1 -C.sub.4烷氧基;X为O、##STR2##或直接链接;R.sup.2和R.sup.3,相同或不同,均为C.sub.1 -C.sub.4烷基,但当X为##STR3##时,R.sup.2和R.sup.3相同。
表征谱图
-
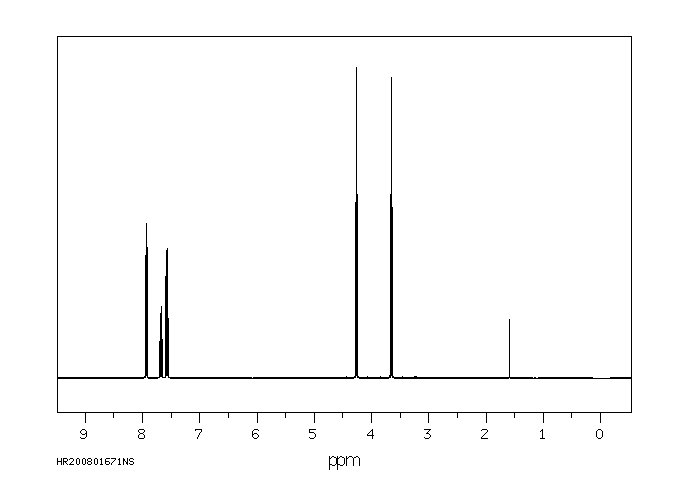
氢谱1HNMR
-
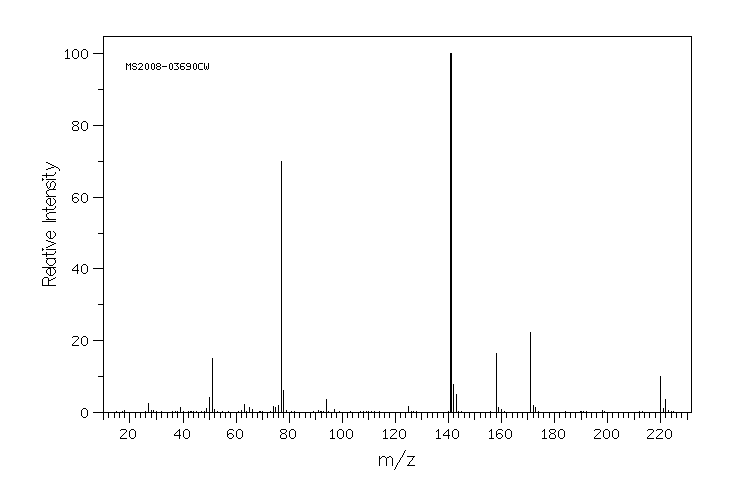
质谱MS
-
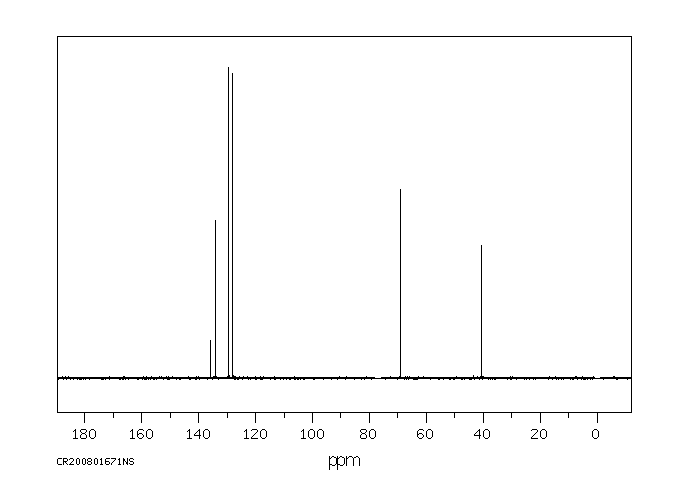
碳谱13CNMR
-
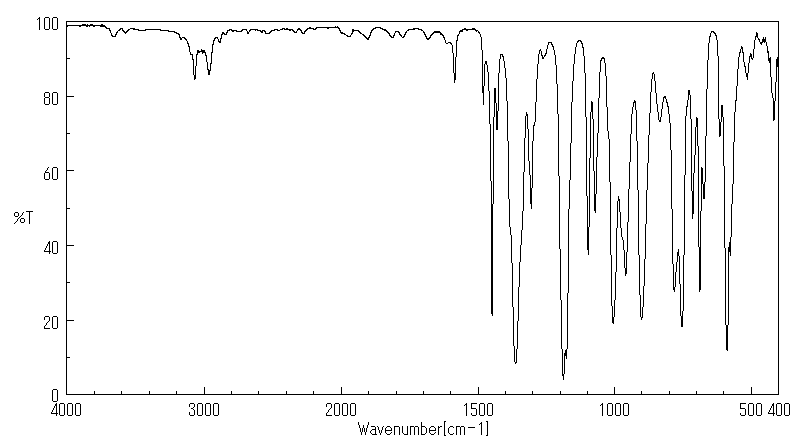
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫