1-(4-氯苯基)-3-苯基-2-硫脲 | 7392-67-8
中文名称
1-(4-氯苯基)-3-苯基-2-硫脲
中文别名
——
英文名称
1-(4-chlorophenyl)-3-phenylthiourea
英文别名
——
CAS
7392-67-8
化学式
C13H11ClN2S
mdl
MFCD00022116
分子量
262.763
InChiKey
XYAKDKSYCSTBMN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:152°C
-
沸点:375.7±44.0 °C(Predicted)
-
密度:1.385±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:56.2
-
氢给体数:2
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
海关编码:2930909090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(4-氯苯)-3-氨基硫脲 4-(p-chlorophenyl)-3-thiosemicarbazide 22814-92-2 C7H8ClN3S 201.68 —— N-(4-chlorophenyl)-N'-phenylurea 1967-26-6 C13H11ClN2O 246.696
反应信息
-
作为反应物:描述:1-(4-氯苯基)-3-苯基-2-硫脲 在 polymer-supported tribromide 作用下, 反应 0.33h, 以81%的产率得到苯并噻唑-2-基-(4-氯-苯基)-胺参考文献:名称:聚合物支撑的三溴化物作为新型固相和可循环使用的催化剂,可在无溶剂微波辐射条件下合成2-(N-芳基氨基)苯并噻唑摘要:2-(N-芳基氨基)苯并噻唑的固相合成是通过使取代的硫脲与聚合物支撑的三溴化物反应来实现的。在微波辐射下高产率地制备了一系列2-(N-芳基)氨基苯并噻唑。该方法具有许多优点,例如反应时间短,产率高以及对环境有益的方法。而且,该催化剂可按常规方法回收并至少重复使用四次而不会损失活性。因此,根据绿色化学惯例合成一些2-(N-芳基氨基)苯并噻唑将是非常令人感兴趣的。DOI:10.1002/jhet.1928
-
作为产物:描述:参考文献:名称:无催化剂和无溶剂条件下基于异氰化物的多组分反应,用于合成不对称硫脲摘要:通过芳族异氰酸酯,胺与1,2-二叔丁基二硫醚(DTBS)的顺序一锅三组分反应,开发了一种新的高效合成硫脲衍生物的方法,并在其中合成了27个不同的实例好到极好的产量。DTBS被认为是不同时使用催化剂和溶剂的有效硫替代物。该协议不使用任何过渡金属催化剂或特殊的实验装置。DOI:10.1016/j.tetlet.2016.11.082
文献信息
-
Synthesis and uncoupling activities of hydrophobic thioureas.作者:SEIJU KUBOTA、KISAKO HORIE、HEMANTK. MISRA、KOUHEI TOYOOKA、MASAYUKI UDA、MASAYUKI SHIBUYA、HIROSHI TERADADOI:10.1248/cpb.33.662日期:——Various N-aryl-N'-phenylthioureas, N, N'-diarylthioureas and N-(1, 2, 4-triazol-3-yl)-N'-arylthioureas were prepared and examined for uncoupling activities. The results indicate that substitution at the 4-position of the phenyl groups of diaryl thioureas is very important for uncoupling activities. Diphenyl thioureas substituted with two or more halogen atoms exhibited strong activities. The highest activity was exhibited by a compound containing nitro groups on both phenyl groups. These results indicate that the hydrophobicity and acidic nature of the compound are of primary importance for uncoupling activities. A remarkable decrease in activity was observed with the thioureas which were substituted with pyridine and 1, 2, 4-triazole rings. The reaction of phenyl isothiocyanate with 3-amino-1, 2, 4-triazole was also studied.
-
Synthesis and Biological Evaluation of Halogenated 2-Arylimino-3-arythiazolidine- 4-ones Containing Benzoic Acid Fragments as Antibacterial Agents作者:Jie Zhang、Likang Zheng、Huayong Liu、Dan Zhao、Di Qu、Shiqing HanDOI:10.2174/15701808113109990031日期:2013.10.1A series of halogenated 2-arylimino-3-ary-thiazolidine-4-ones containing (5-furan-2-yl)-benzoic acid or (5-thiophene-2-yl)-benzoic acid fragments were synthesized, and evaluated in vitro for their antibacterial activity, antibiofilm activity, erythrocyte hemolysis and cytotoxicity. Compounds (7c, 7f, 7i, 7n and 7p) exhibited excellent potency in inhibiting the growth of Staphylococcus epidermidis ATCC35984 (minimal inhibitory concentration (MIC): 0.8 μg/mL). The five compounds also presented promising antibiofilm activity against S. epidermidis ATCC35984 and none of them showed obvious hemolytic activity and cytotoxicity. Further antibacterial effects of the selected five compounds (7c, 7f, 7i, 7n and 7p) against clinical isolates (methicillin-resistant Staphylococcus epidermidis and methicillin-resistant Staphylococcus aureus) were investigated in comparison with methicillin and ampicillin.7p showed the most potent antibacterial activities among the synthesized compounds.合成了包含(5-呋喃-2-基)苯甲酸或(5-噻吩-2-基)苯甲酸片段的一系列卤代2-芳基亚胺-3-芳基-噻唑烷-4-酮,并在体外评估了它们的抗菌活性、抗生物膜活性、红细胞溶血和细胞毒性。化合物(7c、7f、7i、7n和7p)表现出优异的抑制表皮葡萄球菌ATCC35984生长的功效(最小抑制浓度(MIC):0.8 μg/mL)。这五种化合物还显示出对表皮葡萄球菌ATCC35984有前景的抗生物膜活性,并且均未表现出明显的溶血活性和细胞毒性。进一步研究了选定的五种化合物(7c、7f、7i、7n和7p)对临床分离株(耐甲氧西林表皮葡萄球菌和耐甲氧西林金黄色葡萄球菌)的抗菌效果,并与甲氧西林和氨苄西林进行了比较。7p在合成的化合物中显示出最强的抗菌活性。
-
Synthesis and biological evaluation of 2-arylimino-3-pyridin-thiazolineone derivatives as antibacterial agents作者:Ming-Guang Cai、Yang Wu、Jun ChangDOI:10.1016/j.bmcl.2016.03.089日期:2016.5With an intention to find more potent antibacterial agents, four halogen disubstituted thiazolineone derivatives (2a-d), five halogen monosubstituted thiazolineone derivatives (2e-i), and eleven 2-arylimino-3-pyridin-thiazolineone derivatives (2j-t) were synthesized and screened for their antibacterial activity, bactericidal activity, cytotoxicity, and erythrocyte hemolysis. Most of the synthesized
-
Synthetic Studies toward Aryl-(4-aryl-4<i>H</i>-[1,2,4]triazole-3-yl)-amine from 1,3-Diarylthiourea as Urea Mimetics作者:Amarnath Natarajan、Yuhong Guo、Haribabu Arthanari、Gerhard Wagner、Jose A. Halperin、Michael ChorevDOI:10.1021/jo0508189日期:2005.8.14]triazole-3-yl-amines as urea mimetics from the corresponding 1,3-disubstituted thioureas has been studied, and the scope and limitations of this reaction are presented. The reaction proceeds through the formation of a carbodiimide, followed by a sequential addition−dehydration with acyl hydrazides. 1,3-Branched dialkylthioureas result in the formation of the corresponding ureas. The electronic and steric
-
A New Synthetic Protocol for the Preparation of Carbodiimides Using a Hypervalent Iodine(III) Reagent作者:Yunyang Wei、Chenjie Zhu、Dan XuDOI:10.1055/s-0030-1258414日期:2011.3A new, simple, and efficient preparation of symmetrical and unsymmetrical carbodiimides from the corresponding thioureas via dehydrosulfurization using a hypervalent iodine(III) reagent is described. The oxidation afforded carbodiimides in excellent yields and high selectivity. A possible mechanism for the transformation is proposed. desulfurization - hypervalent iodine - oxidations - carbodiimides
表征谱图
-
氢谱1HNMR
-
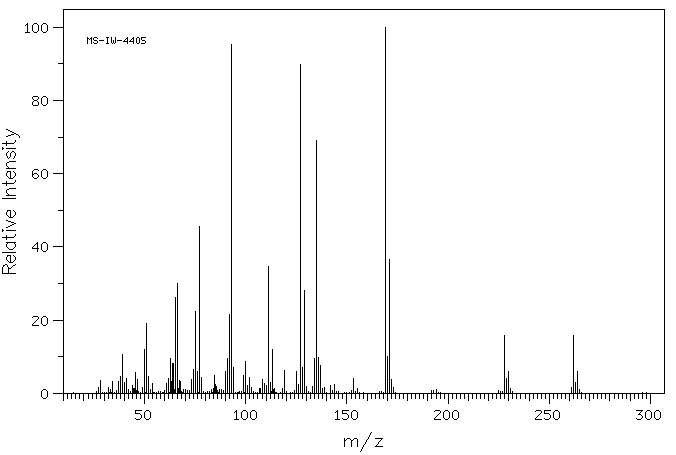
质谱MS
-
碳谱13CNMR
-
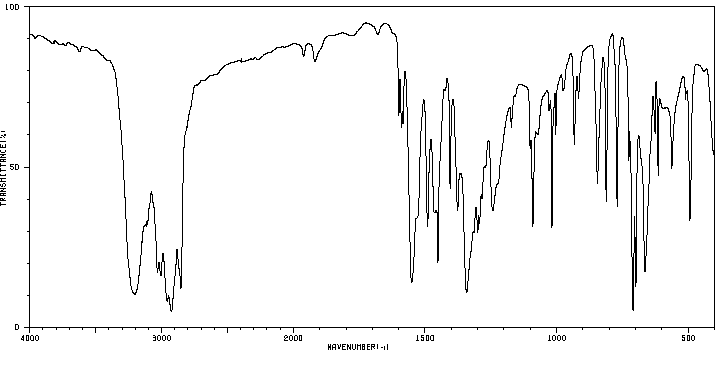
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫