4-肼基喹唑啉 | 36075-44-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:186 °C (decomp)
-
沸点:353.3±25.0 °C(Predicted)
-
密度:1.39±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:63.8
-
氢给体数:2
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
海关编码:2933990090
-
WGK Germany:3
-
危险标志:GHS05,GHS06
-
危险性描述:H301,H318
-
危险性防范说明:P280,P301 + P310,P305 + P351 + P338
-
储存条件:室温
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氨基喹唑啉 4-Aminoquinazoline 15018-66-3 C8H7N3 145.164 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-isopropylidenehydrazinoquinazoline 100061-47-0 C11H12N4 200.243 —— 4-Benzylidenhydrazino-chinazolin 60262-41-1 C15H12N4 248.287 —— 2-chloro-N'-quinazolin-4-ylacetohydrazide —— C10H9ClN4O 236.661 —— 2-quinazolin-4-ylhydrazono-propionic acid 103989-55-5 C11H10N4O2 230.226 —— N-[1-(4-Chloro-phenyl)-meth-(E)-ylidene]-N'-quinazolin-4-yl-hydrazine 129177-07-7 C15H11ClN4 282.732 —— N-[(E)-(4-chlorophenyl)methylideneamino]quinazolin-4-amine —— C15H11ClN4 282.73 —— N-[1-(4-Bromo-phenyl)-meth-(E)-ylidene]-N'-quinazolin-4-yl-hydrazine 129177-05-5 C15H11BrN4 327.183 —— 4-Bromobenzaldehyde 4-quinazolinylhydrazone —— C15H11BrN4 327.18 —— maleic acid (3H-quinazolin-4-ylidene)hydrazide 960216-01-7 C12H10N4O3 258.236 —— 4-oxo-4-(2-(quinazolin-4(3H)-ylidene)hydrazineyl)butanoic acid —— C12H12N4O3 260.252 —— 5-oxo-5-(2-(quinazolin-4(3H)-ylidene)hydrazineyl)pentanoic acid —— C13H14N4O3 274.279 —— 2-[(3H-quinazolin-4-ylidene)hydrazono]propionic acid ethyl ester —— C13H14N4O2 258.28 —— ethyl 2-oxo-2-(2-(quinazolin-4(3H)-ylidene)hydrazineyl)acetate —— C12H12N4O3 260.252 —— N-[1-(2,4-Dichloro-phenyl)-meth-(E)-ylidene]-N'-quinazolin-4-yl-hydrazine 129177-08-8 C15H10Cl2N4 317.177 —— 4-[(2E)-2-(2,4-dichlorobenzylidene)hydrazinyl]quinazoline —— C15H10Cl2N4 317.2 —— N'-quinazolin-4-ylpyridine-3-carbohydrazide —— C14H11N5O 265.274 —— N-(2-(quinazolin-4-yl)hydrazinecarbonothioyl)benzamide 1394045-47-6 C16H13N5OS 323.378 —— 4-methyl-N-(2-(quinazolin-4-yl)hydrazinecarbonothioyl)benzamide 1394045-57-8 C17H15N5OS 337.405 —— 4-chloro-N-(2-(quinazolin-4-yl)hydrazinecarbonothioyl)benzamide 1394045-50-1 C16H12ClN5OS 357.823 —— 4-fluoro-N-(2-(quinazolin-4-yl)hydrazinecarbonothioyl)benzamide 1394045-52-3 C16H12FN5OS 341.369 —— 3-chloro-N-(2-(quinazolin-4-yl)hydrazinecarbonothioyl)benzamide 1394045-49-8 C16H12ClN5OS 357.823 —— 2-methyl-N-(2-(quinazolin-4-yl)hydrazinecarbonothioyl)benzamide 1394045-56-7 C17H15N5OS 337.405 —— N-(2-(quinazolin-4-yl)hydrazinecarbonothioyl)-4-(trifluoromethyl)benzamide 1394045-54-5 C17H12F3N5OS 391.376 —— phenyl[(3H-quinazolin-4-ylidene)hydrazono]acetic acid ethyl ester —— C18H16N4O2 320.351 —— 2-chloro-N-(2-(quinazolin-4-yl)hydrazinecarbonothioyl)benzamide 1394045-48-7 C16H12ClN5OS 357.823 —— 2-fluoro-N-(2-(quinazolin-4-yl)hydrazinecarbonothioyl)benzamide 1394045-51-2 C16H12FN5OS 341.369 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:新系列1,2,4-三唑并[4,3-c]喹唑啉的合成及反应摘要:3-Ethoxycabonyl-1,2,4-triazolo[4,3-c]quinazoline (2) has been synthesized in excellent yield by the condensation of 4-hydrazinoquinazoline (1) with diethyl oxalate and was converted into 3-carbohydrazide 3. The later product was treated with potassium thiocyanate, phenylisothiocyanate or carbon disulfide followed by reaction with hydrazine hydrate to give the respective new 1,2,4-triazolo[4,3-c]quinazoline derivatives 4, 8 or 12. Dehydrative cyclization of the later compounds with concentrated sulphuric acid, sodium hydroxide, m-bromobenzoic acid, carbon disulfide, oxalic acid, phenacyl bromide or benzoin yielded the target heterocycles namely, 1,3,4-thiadiazole, 1,2,4-triazole, 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole or 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazine incorporating 1,2,4-triazolo[4,3-c]quinazoline ring. The structure of the above compounds was confirmed from their spectral characteristics.DOI:10.3987/com-10-12093
-
作为产物:描述:4-羟基喹唑啉 在 tetraphosphorus decasulfide 、 肼 作用下, 以 5,5-dimethyl-1,3-cyclohexadiene 、 异丙醇 为溶剂, 反应 5.0h, 生成 4-肼基喹唑啉参考文献:名称:新型{2-(3-R-1H -1,2,4-三唑-5-基)苯基}胺对抗病原真菌的合成和作用模式研究摘要:由于其高特异性和有效性,三唑类药物已成为治疗人类保健中的真菌感染和控制农业中的植物病原真菌的多功能抗真菌剂。然而,唑类抗性是一个影响人类健康和食品安全的新问题。在这里,我们描述了 10 种新型{2-(3-R-1H-1,2,4-三唑-5-基)苯基}胺的合成。它们的结构由液相色谱-质谱法、1H 和 13C NMR 以及元素分析数据确定。应用体外生长试验,这些三唑类化合物对机会性病原体黑曲霉、12 种真菌(尖孢镰刀菌、藤黑镰刀菌、希金丝炭疽菌、禾谷镰刀菌、禾谷镰刀菌、木炭镰刀菌、赤霉病菌)显示出中等至显着的抗真菌活性禾本科,Verticillium lecanii、Botrytis cinerea、Penicillium digitatum)和三种卵菌(Phytophtora infestans GL-1、P. infestans 4/91;R+ 和 4/91;R-),浓度范围为 1 到 50 µg/mlDOI:10.1002/ardp.201900092
文献信息
-
Quinazoline-containing Hydrazydes of Dicarboxylic Acids and Products of Their Structural Modification: A Novel Class of Anti-inflammatory Agents作者:Nataliia Krasovska、Viktor Stavytskyi、Inna Nosulenko、Oleksandr Karpenko、Oleksii Voskoboinik、Serhii KovalenkoDOI:10.17344/acsi.2020.6440日期:——
The synthesis of hydrazides formed by quinazolin-4(3H)-ylidenehydrazine and dicarboxylic acids, as well as their further modification are described in the present manuscript. It was shown that above-mentioned hydrazides may be obtained via acylation of initial quinazolin-4(3H)-ylidenehydrazine by corresponding acylhalides, cyclic anhydrides and imidazolides of dicarboxylic acids monoesters. Obtained hydrazides were converted into [1,2,4]triazolo[1,5-с]quinazolines that were used as initial compounds for chemical modification aimed to the introduction of amide fragment to the molecule. The IR, 1H NMR and chromato-mass spectral data of obtained compounds were studied and discussed. Obtained substances were studied for anti-inflammatory activity using carrageenan-induced paw inflammation model. Amides of ([1,2,4]triazolo[1,5-с]quinazoline-2-yl)alkyl carboxylic acids were detected as promising class of anti-inflammatory agents for further purposeful synthesis and profound study of anti-inflammatory activity.
本文描述了由喹唑啉-4(3H)-基肼和二羧酸形成的肼类化合物的合成,以及它们的进一步改性。实验证明,上述肼类化合物可以通过对初级喹唑啉-4(3H)-基肼进行酰化反应,使用相应的酰卤、环酐和二羧酸单酯的咪唑酰化物来获得。获得的肼类化合物被转化为[1,2,4]三唑并[1,5-с]喹唑啉,这些化合物被用作化学改性的起始化合物,旨在向分子中引入酰胺基团。对获得的化合物进行了红外光谱、核磁共振和色谱-质谱数据的研究和讨论。使用海藻胶诱导的爪炎模型研究了获得的物质的抗炎活性。检测到([1,2,4]三唑并[1,5-с]喹唑啉-2-基)烷基羧酸酰胺作为有前途的抗炎药物类别,可用于进一步有目的地合成和深入研究抗炎活性。 -
Inhibitors of Apoptosis in Lymphocytes: Synthesis and Biological Evaluation of Compounds Related to Pifithrin-α作者:Sylvie D. Barchéchath、Rommel I. Tawatao、Maripat Corr、Dennis A. Carson、Howard B. CottamDOI:10.1021/jm0502034日期:2005.10.139 were more protective than 39, while the aromatic analogues of 1 were not active. Compound 19 containing a pyrrolidinyl substituent on the phenyl ring provided potent antiapoptotic activity (EC50 of 1.31 microM compared to 4.16 microM for 1). Modification of aromatic 39 with a pyrrolidinyl para substituent (compound 60) enhanced the activity, lowering the EC50 to 0.35 microM. Also, 60 provided significant化学性保护细胞免受毒素或电离辐射诱导的细胞凋亡可能对生物防御和急性损伤的治疗很重要。我们描述了一系列小的杂环,包括稠合的苯并噻唑,苯并咪唑和相关化合物,它们消除了地塞米松和γ射线诱导的胸腺细胞凋亡。为了优化以前报道的pifithrin-alpha(PFT-alpha,1)的保护活性,合成了该衍生物和相应的闭环咪唑并苯并噻唑(IBT,39)的各种衍生物和类似物。39的芳香族类似物比39更具保护性,而1的芳香族类似物则没有活性。在苯环上包含吡咯烷基取代基的化合物19提供了有效的抗凋亡活性(EC50为1.31 microM,而4的EC16为4.16 microM)。用吡咯烷基对位取代基(化合物60)修饰芳香族化合物39可增强活性,将EC50降低至0.35 microM。同样,60预期也提供了有效的保护,以抵抗伽马射线辐射诱导的细胞凋亡。化合物19和60对于潜在的临床开发可能很有希望。
-
Synthesis of 6-N-R-Tetrazolo[1,5-c]quinazolin-5(6H)-ones and Their Anticancer Activity作者:Oleksii Antypenko、Sergiy Kovalenko、Bakhtiyor Rasulev、Jerzy LeszczynskiDOI:10.17344/acsi.2016.2464日期:2016.9.15N-alkylation, are discussed, including reactions with secondary and tertiary amides. Four new synthesized compounds (2.7, 3.2, 5.2, 5.3) were tested in vitro for anticancer activity at 10 μM against 60 cell lines of nine different cancer types: leukemia, melanoma, lung, colon, CNS, ovarian, renal, prostate, and breast cancers. Further synthesis of substances within the series of substituted tetrazolo[1,5-c]quinazoline
-
AZA-ARYL 1H-PYRAZOL-1-YL BENZENE SULFONAMIDES申请人:ChemoCentryx, Inc.公开号:US20180009797A1公开(公告)日:2018-01-11Compounds are provided that act as potent antagonists of the CCR(9) receptor for treating Sjogren's syndrome. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions.提供了作为治疗Sjogren综合征的CCR(9)受体的有效拮抗剂的化合物。这些化合物通常是芳基磺酰胺衍生物,并且在制药组合物中很有用。
-
Metal free [4+1] and [5+1] annulation reactions to prepare heterocycles using DMF and its derivatives as one-carbon source作者:Yiwen Xu、Bei Shen、Lingfeng Liu、Chunhua QiaoDOI:10.1016/j.tetlet.2020.151844日期:2020.5in a variety of pharmaceutical and agrochemical agents. Herein, we report a highly efficient and practical method using DMF and its derivative for the [4+1] and [5+1] annulation reactions to prepare these heterocycles. This metal free reaction takes advantages of shelf stable DMF as solvent and carbon donor, imidazole chloride as catalyst, the mild reaction condition tolerates a broad substrate range
表征谱图
-
氢谱1HNMR
-
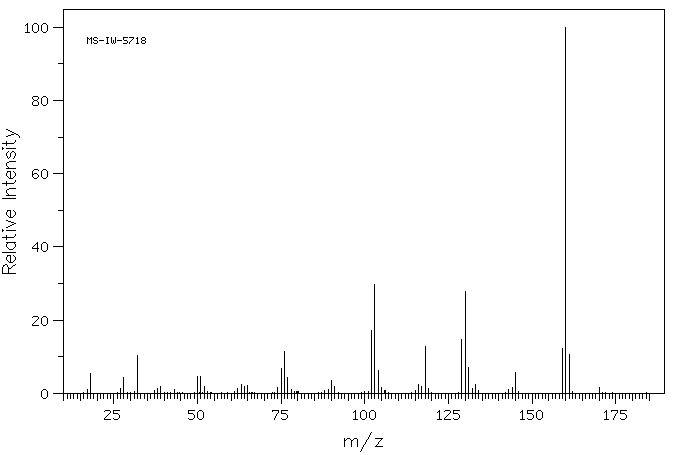
质谱MS
-
碳谱13CNMR
-
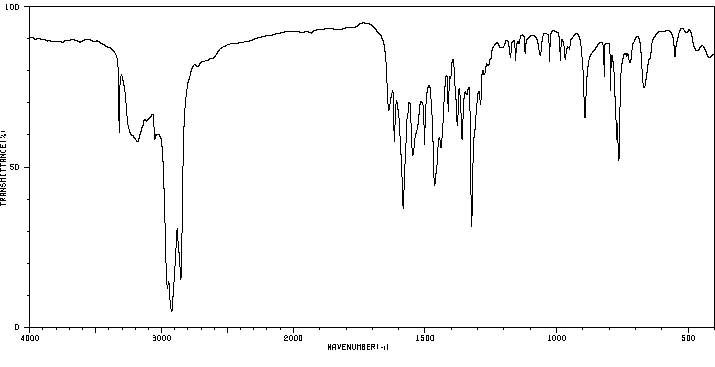
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息