3-乙基联苯 | 5668-93-9
中文名称
3-乙基联苯
中文别名
——
英文名称
3-ethyl-1,1'-biphenyl
英文别名
3-ethylbiphenyl;ethylbiphenyl;3-ethyl-biphenyl;3-Aethyl-biphenyl;3-Aethylbiphenyl;m-Aethylbiphenyl;1-ethyl-3-phenylbenzene
CAS
5668-93-9
化学式
C14H14
mdl
MFCD00796638
分子量
182.265
InChiKey
HUXKTWJQSHBZIV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-27 °C
-
沸点:284 °C
-
密度:1.00
-
保留指数:1628;1621;1633
-
稳定性/保质期:
在常温常压下,该物质保持稳定。
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902909090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将药品存放在避光、阴凉干燥处,并密封保存。
SDS
3-乙基联苯 修改号码:5
模块 1. 化学品
产品名称: 3-Ethylbiphenyl
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3-乙基联苯
百分比: >98.0%(GC)
CAS编码: 5668-93-9
分子式: C14H14
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
3-乙基联苯 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点:
-27°C
沸点/沸程 284 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.00
溶解度:
[水] 无资料
[其他溶剂] 无资料
3-乙基联苯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
3-乙基联苯 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 3-Ethylbiphenyl
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3-乙基联苯
百分比: >98.0%(GC)
CAS编码: 5668-93-9
分子式: C14H14
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
3-乙基联苯 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点:
-27°C
沸点/沸程 284 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.00
溶解度:
[水] 无资料
[其他溶剂] 无资料
3-乙基联苯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
3-乙基联苯 修改号码:5
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:铁催化的苄基 C(sp3)–H 键的分子间胺化摘要:利用 1,2,3,4-四唑作为氮烯前体,通过铁催化开发了分子间苄基 C(sp 3 )–H 胺化催化体系。该方法能够将 2-氨基吡啶直接安装到苄基和杂苄基位置。该方法选择性地胺化 2° 苄基 C(sp 3 )–H 键,超过 3° 和 1° 苄基 C(sp 3 )–H 键。实验研究表明,C(sp 3 )–H 胺化是通过形成苄基自由基中间体进行的。本研究报告了使用廉价、生物相容性贱金属催化合成 2-吡啶取代的苄胺的新方法的发现,该方法应在药物化学和药物发现方面具有广泛的应用。DOI:10.1021/jacs.2c10719
-
作为产物:参考文献:名称:通过光实现无过渡金属的 CC、CO 和 CN 交叉耦合摘要:用于构建 CC、CO 和 CN 键的过渡金属催化交叉偶联已经彻底改变了化学科学。尽管取得了巨大的成就,但这些金属催化剂也存在一些问题,包括成本高、需要专门的配体、对空气和水分的敏感性以及所谓的“过渡金属残留问题”。不依赖于成熟的氧化加成、金属转移和还原消除机制范式的互补策略可能会消除所有这些与金属相关的问题。在此,我们表明芳基三氟甲磺酸酯可以与芳基三氟硼酸钾、脂肪醇和腈偶联,而无需借助光能赋能的金属催化剂。对照实验表明,在所有常见的芳基亲电试剂中,只有芳基三氟甲磺酸酯能够进行这些偶联,而芳基碘化物和溴化物不能作为偶联伙伴。DFT 计算表明,一旦转化为芳基自由基阳离子,芳基三氟甲磺酸酯将更有利于 ipso 取代。荧光光谱和循环伏安法研究表明,激发态丙酮和芳基三氟甲磺酸酯之间的相互作用对于这些偶联是必不可少的。预计本报告中的结果将为执行交叉耦合提供新的机会。荧光光谱和循环伏安法研究表明,激发态DOI:10.1021/jacs.9b02684
文献信息
-
Equilibria of isomeric transformations of alkylbiphenyls作者:I.Yu Roshchupkina、T.N Nesterova、A.M RozhnovDOI:10.1016/0021-9614(87)90137-6日期:1987.3Abstract Equilibria of mutual transformations of mono-, di-, and tri-alkylbiphenyls ( abp ) were investigated in the liquid phase at 308 to 423 K. On the basis of experimental equilibrium constants, values of ΔrHmo/(kJ·mol−1) and ΔrSmo/(J·K−1·mol−1) were calculated. Below are given correspondingly: reaction, compound and values for Et- bp (I), i-Pr- bp (II), and t-Bu- bp (III): 4- abp = 3- abp , I摘要 研究了液相中单、二和三烷基联苯 ( abp ) 相互转化的平衡,温度为 308 至 423 K。根据实验平衡常数,ΔrHmo/(kJ·mol−1) 值和ΔrSmo/(J·K-1·mol-1)被计算。下面分别给出:Et-bp (I)、i-Pr-bp (II) 和 t-Bu-bp (III) 的反应、化合物和值: 4- abp = 3- abp , I, 0.23, 5.76 ; 二、(0.45±0.41)、(5.72±1.13);三、(0.48±0.53)、(4.83±0.53);2- abp = 4- abp , I, -3.3, -5.76; II、-12.6、-5.76;III、-15.4、-5.76;3,5-di-abp = 3,3'-di-abp, I, -0.1, 5.76; 二、(0±0.60)、(5.98±1.65);三、(-1.34±0.67)、(4.48±1.87);3
-
Lewis Acid Assisted Nickel‐Catalyzed Cross‐Coupling of Aryl Methyl Ethers by C−O Bond‐Cleaving Alkylation: Prevention of Undesired β‐Hydride Elimination作者:Xiangqian Liu、Chien‐Chi Hsiao、Indrek Kalvet、Matthias Leiendecker、Lin Guo、Franziska Schoenebeck、Magnus RuepingDOI:10.1002/anie.201510497日期:2016.5.10In the presence of trialkylaluminum reagents, diverse aryl methyl ethers can be transformed into valuable products by C−O bond‐cleaving alkylation, for the first time without the limiting β‐hydride elimination. This new nickel‐catalyzed dealkoxylative alkylation method enables powerful orthogonal synthetic strategies for the transformation of a variety of naturally occurring and easily accessible anisole
-
Visible-Light-Driven Organocatalytic Alkoxylation of Benzylic C–H Bonds作者:Chunbo Bo、Fei Chen、Qingqing Bu、Zhi-Hong Du、Min Li、Bin Dai、Ning LiuDOI:10.1021/acs.joc.2c02743日期:2023.3.17A variety of strategies for direct alkoxylation of the benzyl C–H bond have been developed toward the construction of benzyl ethers. The light-induced benzyl C–H bond alkoxylation provides an alternative strategy for the synthesis of these important intermediates. The photocatalyzed alkoxylation of the benzyl C–H bond has dominated by metal-catalyzed methods. Herein, we reported a light-driven organocatalytic
-
Site‐Selective Pyridination of Benzylic and Allylic C−H bonds via Radical‐Radical Cross‐Coupling作者:Jun‐Jie Chen、Zhan‐Jie Wang、Huan‐Ming HuangDOI:10.1002/adsc.202301461日期:2024.3.19bonds. The site-selective C―H pyridination could successfully couple benzylic/allylic C―H bonds with pyridylphosphonium salts, which installed directly and regioselectively from C―H heteroarenes through a radical-radical cross coupling mechanism. This synthetic methodology could tolerance a variety of functional groups, complex heteroarenes, even late-stage functionalization of pharmaceuticals selectively
-
Palladium-Catalyzed Arylation of Simple Arenes with Iodonium Salts作者:Thomas E. Storr、Michael F. GreaneyDOI:10.1021/ol400412z日期:2013.3.15The development of an arylation protocol for simple arenes with diaryliodonium salts using the Herrmann-Beller palladacycle catalyst is reported. The reaction takes simple aromatic feedstocks and creates valuable biaryls for use in all sectors of the chemical industry.
表征谱图
-
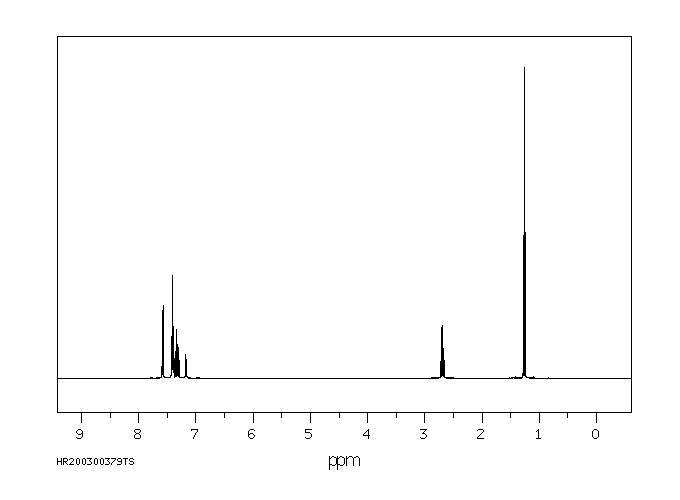
氢谱1HNMR
-
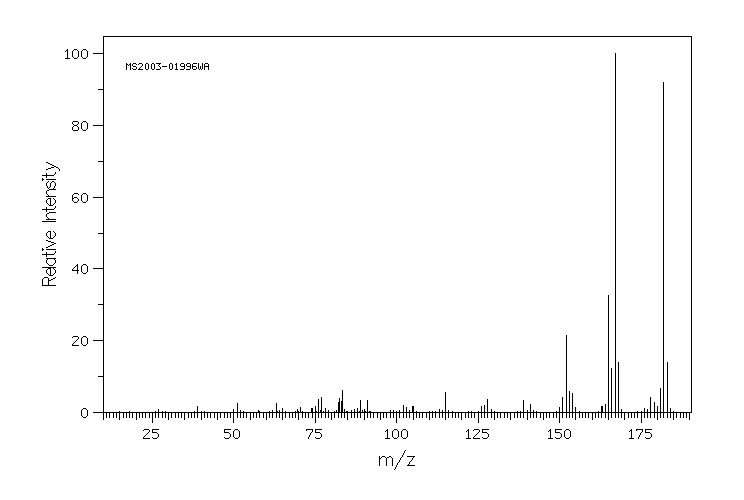
质谱MS
-
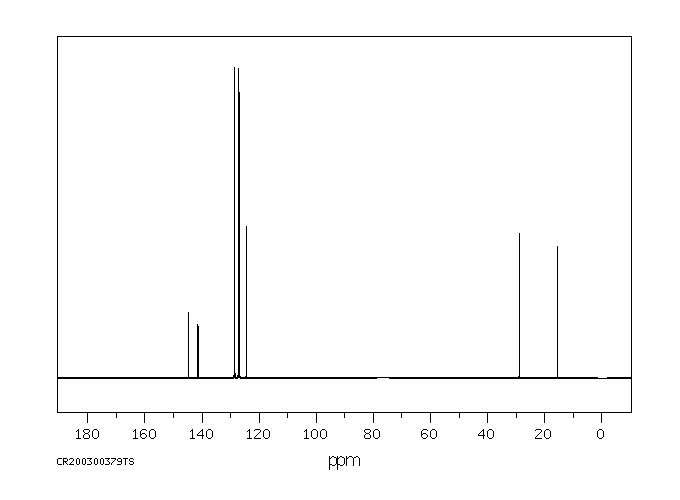
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫