1,2-二甲基-3-羟基-4-吡啶酮 | 30652-11-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:272-275 °C (lit.)
-
沸点:255.1°C (rough estimate)
-
密度:1.1997 (rough estimate)
-
溶解度:溶于水(加热时高达5mg/ml)。
-
颜色/状态:Needles from water
-
味道:Very bitter taste
-
蒸汽压力:1.56X10-4 mm Hg at 25 °C (est)
-
稳定性/保质期:
常温常压下稳定。
-
解离常数:pKa1 = 3.3; pKa2 = 9.7
-
碰撞截面:123.2 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:40.5
-
氢给体数:1
-
氢受体数:3
ADMET
安全信息
-
危险品标志:Xn
-
安全说明:S26,S36
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2933399090
-
RTECS号:UU7785940
-
危险标志:GHS07
-
危险性描述:H302,H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。确保工作环境有良好通风或排气设施。
SDS
: 1,2-二甲基-3-羟基-4-吡啶酮
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P330 漱口。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
当心 - 物质尚未完全测试。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C7H9NO2
分子式
: 139.15 g/mol
分子量
组分 浓度或浓度范围
4(1H)-Pyridinone, 1,2-dimethyl-3-hydroxy-
-
CAS 号 30652-11-0
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 272 - 275 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 2,000 mg/kg
备注: 心脏的:其他改变。
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
附加说明
化学物质毒性作用登记: UU7785940
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
去铁酮(Deferiprone)是一种铁螯合剂,以3:1(配体:铁)的摩尔比结合铁,用于治疗依赖输血的地中海贫血。每日剂量为每公斤体重100毫克,并且能够穿过血脑屏障。
作为一种脂溶性铁螯合剂,去铁酮通过与铁结合,从体内去除多余的铁,有效促进铁排除,阻止输血依赖的地中海贫血患者血清铁负荷的蓄积。它不仅具有铁螯合剂的作用,还具备保护作用。
药理作用去铁酮是继去铁胺之后应用于临床的祛铁剂,也是第一个口服的铁螯合剂,能够有效促进铁排除,阻止输血依赖的地中海贫血患者血清铁负荷的蓄积。体内和体外研究均表明,DFP能去除细胞内铁、网状内皮系统内铁、转铁蛋白结合铁及以铁蛋白和含铁血黄素形式储存的铁。多项前瞻性和回顾性研究均证明DFP治疗重型地中海贫血患者心脏铁过载疗效明显优于DFO。
药动学去铁酮(DFP)口服给药后经消化很快被吸收,空腹给药45-60分钟达到峰浓度,餐后服用则达峰时间延长至2小时。尽管与食物同服并不降低吸收总量,但餐后服用的血清峰浓度比空腹服用低。
DFP半衰期为2-3小时,经葡萄糖醛酸化代谢失活,主要通过肾脏清除,在服用后的24小时内总剂量的75%~90%随尿排出,以原形、葡萄糖醛酸化代谢产物以及铁-去铁酮复合物的形式存在。有研究表明,在肾功能不全的人群中,DFP的肾脏清除率下降,但全身清除率并未下降。
临床应用用于不耐受或不愿意接受现有螯合剂治疗的铁负荷过多的地中海贫血患者。
不良反应去铁酮常见的不良反应包括关节痛(尤其是大关节)、一过性的丙氨酸转氨酶升高、胃肠道反应和锌缺乏。关节痛症状在DFP减量和应用非甾体类抗炎药物后仍未缓解者应停药。严重的不良反应是粒细胞减少和粒细胞缺乏,建议每周至少检测1次血常规。若出现粒细胞减少症应暂停使用,若出现粒细胞缺乏症则从此禁用。
生物活性去铁酮(CP20)是一种螯合剂,具有对铁离子的亲和性(三价铁离子),与铁离子结合形成中性的3:1 (去铁铜:铁)复合物。
体外研究在兔子体内,去铁酮(100毫克/千克)降低平均基底动脉截面积24%,并表现出内弹性膜褶皱的可变数量。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2-二甲基-3-苯基甲氧基吡啶-4-酮 1,2-dimethyl-3-(benzyloxy)-4(1H)-pyridinone 90037-22-2 C14H15NO2 229.279 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-乙基-3-羟基-2-甲基吡啶-4-酮 CP21 30652-12-1 C8H11NO2 153.181 3-羟基-1-(2-羟基乙基)-2-甲基吡啶-4-酮 CP40 30652-21-2 C8H11NO3 169.18 1-丁基-3-羟基-2-甲基吡啶-4-酮 1,n-butyl-3-hydroxy-2-methylpyrid-4-one 30652-15-4 C10H15NO2 181.235 —— [Ga(dmpp)3] 123923-62-6 C21H24GaN3O6 484.161 —— 3-Ethoxycarbonyloxy-1,2-dimethyl-4(1H)-pyridinone —— C10H13NO4 211.218 —— 1,2-dimethyl-3-pivaloyloxy-4(1H)-pyridinone —— C12H17NO3 223.272 3-羟基-1,2-二甲基吡啶-4-硫酮 3-hydroxy-1,2-di-methylpyridine-4(1H)-thione 209248-75-9 C7H9NOS 155.221 —— 3-(diethylaminocarbonyloxy)-1,2-dimethyl-4(1H)-pyridinone —— C12H18N2O3 238.287 —— 3-Diphenylboranyloxy-1,2-dimethyl-pyridin-4-one —— C19H18BNO2 303.2
反应信息
-
作为反应物:描述:参考文献:名称:与生物有关的O,S-供体化合物。合成,钼络合和黄嘌呤氧化酶抑制摘要:两个O,S-供体配体,羟基硫吡喃酮 和 羟基硫代吡啶酮 开发和研究了衍生物,以及相应的 Ø,Ô衍生物,考虑到其潜在的药理应用,如黄嘌呤氧化酶(XO) 抑制剂。生物学分析表明,O,S-配体对XO具有很高的抑制活性(纳摩尔级,接近于XO)。制药业 药品 别嘌醇),与相应的O,O-类似物相反。由于这种生物医学意义钼含 酵素, 相应的 钼(VI)对复合物进行了溶液和固态研究,旨在鉴定其生物学特性的来源。解决方案研究表明,与O,O-类似物相比,钼(VI)与O,S-配体形成的配合物表现出一定的稳定性,对于还原钼(IV)物种。的晶体结构钼(VI) 与 羟基硫吡喃酮揭示了配位模式的良好灵活性,分别为单核和双核物种具有两种结构异构体和两种多晶型形式。这些结果为涉及XO相互作用的XO抑制的机理建议提供了支持。硫酮 团体 与 钼辅因子,从而表明 硫 原子在XO上有抑制作用。DOI:10.1039/b717172b
-
作为产物:描述:3-(苄氧基)-2-甲基-4H-吡喃-4-酮 在 palladium on activated charcoal 氢气 作用下, 以 甲醇 、 乙醇 为溶剂, 反应 24.0h, 生成 1,2-二甲基-3-羟基-4-吡啶酮参考文献:名称:3-Hydroxy-4-pyrones as Precursors of 4-Methoxy-3-oxidopyridinium Ylides. An Expeditious Entry to Highly Substituted 8-Azabicyclo[3.2.1]octanes摘要:3-Hydroxy-4-pyridones, which are easily prepared from commercially available 3-hydroxy-4-pyrones, can be readily transformed into 4-methoxy-3-oxidopyridinium ylides by treatment with methyl trifluoromethanesulfonate and subsequent deprotonation with a non-nucleophilic base. These ylides are capable of undergoing cycloaddition to several electron-deficient alkenes, thus allowing the synthesis of highly functionalized azabicyclo[3.2,1]octane moieties. The rich substitution patterns of these frameworks might allow their divergent conversion to a variety of natural and non-natural tropane alkaloids.DOI:10.1021/jo960854v
文献信息
-
[EN] NOVEL HEPCIDIN MIMETICS AND USES THEREOF<br/>[FR] NOUVEAUX MIMÉTIQUES D'HEPCIDINE ET LEURS UTILISATIONS申请人:BAYER HEALTHCARE LLC公开号:WO2018128828A1公开(公告)日:2018-07-12The present invention relates to novel peptides acting as hepcidin mimetics, as well as analogues and derivatives thereof. The invention further relates to compositions comprising the peptides of the present invention, and to the use of the peptides in the prophylaxis and treatment of hepcidin-associated disorders, including prophylaxis and treatment of iron overload diseases such as hemochromatosis, iron-loading anemias such as thalassemia, and diseases being associated with ineffective or augmented erythropoiesis, as well as further related conditions and disorders described herein.
-
TARGETED IONOPHORE-BASED METAL DELIVERY申请人:The Regents of the University of California公开号:US20200113937A1公开(公告)日:2020-04-16The present disclosure provides ionophore compounds, which are useful for facilitating delivery of a metal ion to a cell, tissue or organ of interest. The present disclosure provides compositions comprising the subject ionophore compounds. The present disclosure provides methods of delivering a metal ion intracellularly to a target cell. The present disclosure also provides methods of treating a condition associated with a metal deficiency in an individual.
-
[EN] LYMPHATIC SYSTEM-DIRECTING LIPID PRODRUGS<br/>[FR] PROMÉDICAMENTS LIPIDIQUES ORIENTANT VERS LE SYSTÈME LYMPHATIQUE申请人:ARIYA THERAPEUTICS INC公开号:WO2019046491A1公开(公告)日:2019-03-07The present invention provides lymphatic system-directing lipid prodrugs, pharmaceutical compositions thereof, methods of producing such prodrugs and compositions, as well as methods of improving the bioavailability or other properties of a therapeutic agent that comprises part of the lipid prodrug. The present invention also provides methods of treating a disease, disorder, or condition such as those disclosed herein, comprising administering to a patient in need thereof a provided lipid prodrug or a pharmaceutical composition thereof.本发明提供了淋巴系统定向脂质前药,其制药组合物,制备这种前药和组合物的方法,以及改善作为脂质前药一部分的治疗剂的生物利用度或其他性质的方法。本发明还提供了治疗疾病、紊乱或症状的方法,包括向需要的患者施用所提供的脂质前药或其制药组合物。
-
SUBSTITUTED 2,4 DIAMINO-QUINOLINE AS NEW MEDICAMENT FOR FIBROSIS, AUTOPHAGY AND CATHEPSINS B (CTSB), L (CTSL) AND D (CTSD) RELATED DISEASES申请人:Genoscience Pharma SAS公开号:EP3620164A1公开(公告)日:2020-03-11The present invention relates to novel 2-primary amino-4-secondary amino-quinoline derivatives, their manufacture, pharmaceutical compositions comprising them and their use as medicaments. The active compounds of the present invention can be useful as a medicament in the treatment and/or the decreasing and/or the prevention of fibrosis and/or fibrosis related diseases, or for use as a medicament in the treatment and/or the decreasing and/or the prevention of the autophagy and/or autophagy related diseases and for the inhibition of the autophagy flux, or for use in the inhibition of cathepsins B (CTSB), L (CTSL) and/or D (CTSD) and/or cathepsins B (CTSB), L (CTSL) and/or D (CTSD) related diseases; with the proviso that said compounds are not to be used for the treatment of any forms of cancers.
-
[EN] COMBINATION OF HEPATITIS B VIRUS (HBV) VACCINES AND PYRIDOPYRIMIDINE DERIVATIVES<br/>[FR] ASSOCIATION DE VACCINS CONTRE LE VIRUS DE L'HÉPATITE B (VHB) ET DE DÉRIVÉS DE PYRIDOPYRIMIDINE申请人:JANSSEN SCIENCES IRELAND UNLIMITED CO公开号:WO2020255038A1公开(公告)日:2020-12-24Therapeutic combinations of hepatitis B virus (HBV) vaccines and a pyridopyrimidine derivative are described. Methods of inducing an immune response against HBV or treating an HBV-induced disease, particularly in individuals having chronic HBV infection, using the disclosed therapeutic combinations are also described. The invention provides therapeutic combinations or compositions and methods for inducing an immune response against hepatitis B viruses (HBV) infection.
表征谱图
-
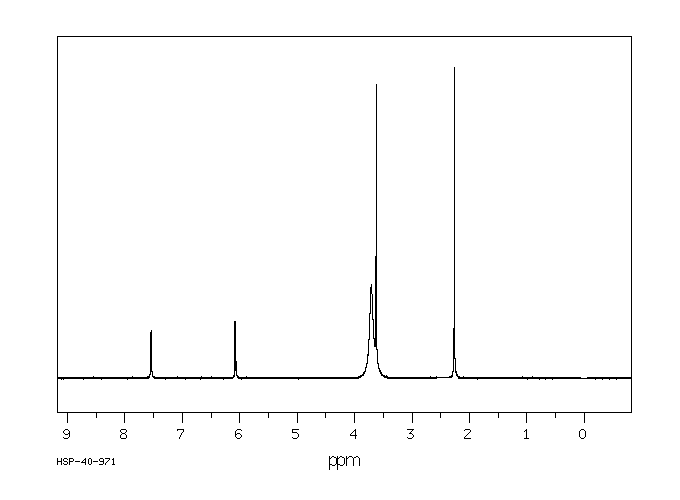
氢谱1HNMR
-
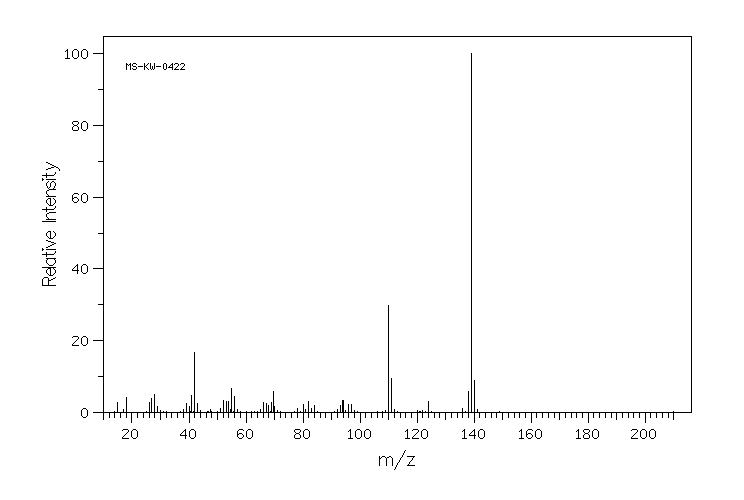
质谱MS
-
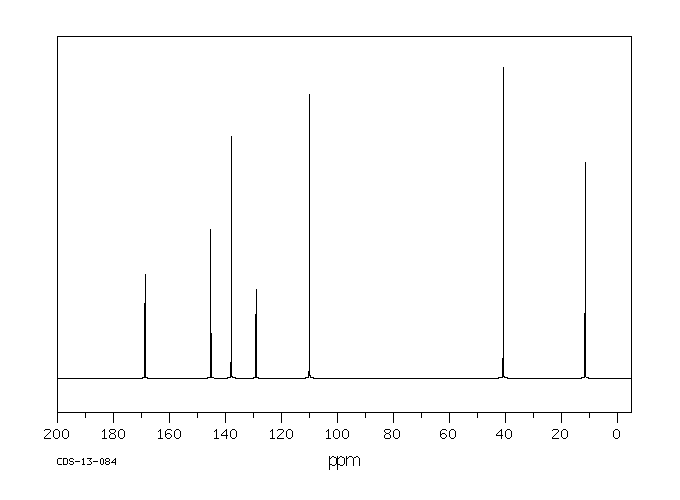
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息