3-甲氧基-2(1H)-吡啶酮 | 20928-63-6
中文名称
3-甲氧基-2(1H)-吡啶酮
中文别名
2-羟基-3-甲氧基吡啶;3-甲氧基-2(1H)-羟基吡啶;3-甲氧基-2-羟基吡啶
英文名称
3-methoxy-1H-pyridin-2-one
英文别名
3-methoxypyridin-2(1H)-one;3-methoxy-2-pyridone;3-methoxy-2(1H)-pyridone;3-methoxy-2(1H)-pyridinone;3-methoxypyridin-2-one;3-methoxy-1,2-dihydro-pyridin-2(1H)-one
CAS
20928-63-6
化学式
C6H7NO2
mdl
MFCD00006015
分子量
125.127
InChiKey
LKIMDXQLHFCXQF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:115-117 °C(lit.)
-
沸点:354.2±42.0 °C(Predicted)
-
密度:1.16±0.1 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:38.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn
-
安全说明:S26
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2933399090
-
危险标志:GHS07
-
危险性描述:H302,H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:将贮藏器密封后,放入一个紧密的容器中,并存放在阴凉、干燥的地方。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Hydroxy-3-methoxypyridine
Synonyms: 3-Methoxypyridin-2-ol
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H302: Harmful if swallowed
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 2-Hydroxy-3-methoxypyridine
CAS number: 20928-63-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
No data
Boiling point:
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C6H7NO2
Molecular weight: 125.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Hydroxy-3-methoxypyridine
Synonyms: 3-Methoxypyridin-2-ol
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H302: Harmful if swallowed
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 2-Hydroxy-3-methoxypyridine
CAS number: 20928-63-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
No data
Boiling point:
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C6H7NO2
Molecular weight: 125.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 吡啶-2,3-二醇 dihydroxypyridine 16867-04-2 C5H5NO2 111.1 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-methyl-3-methoxypyridin-2-one 54955-13-4 C7H9NO2 139.154 —— 3-methoxy-5-methylpyridin-2(1H)-one —— C7H9NO2 139.154 5-溴-3-甲氧基吡啶-2(1H)-酮 5-bromo-3-methoxypyridin-2(1H)-one 1189757-62-7 C6H6BrNO2 204.023 —— 6-bromo-3-methoxypyridin-2-ol 1416800-99-1 C6H6BrNO2 204.023 1-甲基-3-羟基-2(1H)-吡啶酮 1-methyl-3-hydroxy-2(1H)-pyridinone 19365-01-6 C6H7NO2 125.127 —— 1-propargyl-3-methoxypyridin-2-one 1416915-15-5 C9H9NO2 163.176
反应信息
-
作为反应物:描述:3-甲氧基-2(1H)-吡啶酮 在 正丁基锂 、 2,6-二叔丁基-4-甲基苯酚 、 甲基锂 作用下, 以 二氯甲烷 为溶剂, 反应 82.33h, 生成 dimethyl 1β-methoxy-4α-<(4'-methylbenzenesulfonyl)amino>cyclohex-5-ene-1,2-dicarboxylate参考文献:名称:Diels-Alder cycloadditions using nucleophilic 2-pyridones. Regiocontrolled and stereocontrolled synthesis of unsaturated, bridged, bicyclic lactams摘要:Captodative 3-oxy-and 3-(tolylthio)-1-tosyl-2-pyridones 1a-1d are shown to be reactive as nucleophilic dienes undergoing 2 + 4-cycloadditions with various electrophilic alkenes under sufficiently mild thermal conditions (90-100-degrees-C) that the initial bicylic lactam adducts can be isolated on gram scale in fair to very good yields (23-83%) without loss of an isocyanate from the heteroatom bridge. These bicyclic adducts are formed with complete regiocontrol and stereocontrol. For pyridone sulfide 1d, these Diels-Alder cycloadditions are the first examples of a captodative unsaturated sulfide acting as an enophile. NMR data (C-13) are presented correlating the electron density in the pyridone diene systems with their Diels-Alder reactivity, and some transformations of the bicyclic lactam adducts are shown to illustrate the value and versatility of these richly functionalized synthetic intermediates.DOI:10.1021/jo00041a010
-
作为产物:描述:2-溴-3-甲氧基吡啶 在 copper(l) iodide 、 C14H14N2O3 、 potassium hydroxide 作用下, 以 环丁砜 、 水 为溶剂, 以95 %的产率得到3-甲氧基-2(1H)-吡啶酮参考文献:名称:使用羟基吡啶甲酰胺配体的铜催化芳基卤化物的羟基化摘要:卤代芳烃的羟基化是有机合成化学中的一个基本转变。羟基吡啶甲酰胺配体能够实现杂芳基卤化物的有效铜催化羟基化,并具有广泛的官能团耐受性。 Cu-MPBS 系统最初设计用于 C-N 偶联,可实现铜催化芳基溴的羟基化。相关衍生物 Cu-HMPS 为芳基溴、芳基碘和活化芳基氯的羟基化提供了出色的反应性和纯度。邻位活化底物对铜催化的羟基化表现出极高的反应活性和选择性。更困难的芳基氯化物,需要更高活化温度(120°C)的底物,可以通过具有优异的内在配体稳定性的Cu-DMPS系统进行羟基化。可以通过配体、溶剂和碱基的选择来调整反应条件以适应目标底物。采用 KOH、K 2 CO 3 或 K 3 PO 4 水溶液(溶于环丁砜或环丁砜)设计了安全可靠的加工条件和酒精混合物。DOI:10.1021/acs.oprd.4c00108
文献信息
-
Substituted Heteroaromatic Pyrazole-Containing Carboxamide and Urea Compounds as Vanilloid Receptor Ligands申请人:FRANK Robert公开号:US20130029962A1公开(公告)日:2013-01-31Substituted heteroaromatic pyrazole-containing carboxamide and urea compounds as vanilloid receptor ligands, pharmaceutical compositions containing these compounds and also to a method of using these compounds for treating and/or inhibiting pain and further diseases and/or disorders.
-
PYRROLO AND PYRAZOLOPYRIMIDINES AS UBIQUITIN-SPECIFIC PROTEASE 7 INHIBITORS申请人:Forma Therapeutics, Inc.公开号:US20160185785A1公开(公告)日:2016-06-30The invention relates to inhibitors of USP7 inhibitors useful in the treatment of cancers, neurodegenerative diseases, immunological disorders, inflammatory disorders, cardiovascular diseases, ischemic diseases, viral infections and diseases, and bacterial infections and diseases, having the Formula: where m, n, X 1 , X 2 , R 1 -R 5 , R 5′ and R 6 are described herein.
-
Synthesis and iron chelating properties of hydroxypyridinone and hydroxypyranone hexadentate ligands作者:Tao Zhou、Xiao-Le Kong、Robert C HiderDOI:10.1039/c8dt05014g日期:——chelators, four hexadentate ligands were synthesized by conjugating the corresponding bidentate ligands (3-hydroxypyridin-4-one (3,4-HOPO), 3-hydroxypyridin-2-one (3,2-HOPO), 1-hydroxypyridin-2-one (1,2-HOPO), and 3-hydroxypyran-4-one) each with a free amino group to a tripodal acid. Their pKa values and affinities for iron(III) were investigated. The pFe3+ values of the hexadentate pyridinones 1 (3螯合疗法已成为治疗某些疾病的重要方法。为了确定临床上有用的螯合剂,通过缀合相应的二齿配体(3-羟基吡啶-4-酮(3,4-HOPO),3-羟基吡啶-2-酮(3,2-HOPO), 1-羟基吡啶-2-酮(1,2-HOPO)和3-羟基吡喃-4-酮)各自具有与三脚架酸形成的游离氨基。研究了它们对铁(III)的p K a值和亲和力。该PFE 3+的六齿吡啶酮的值1(3,4- HOPO),3(3,2-HOPO)和4(1,2- HOPO)和吡喃酮2被发现跟随序列1 >4 » 3 > 2,这是对PFE不同3+的相应的二齿形式(3,4- HOPO»3,2-HOPO> 1,2- HOPO> 3- hydroxypyranone)值序列。六齿的3,4-HOPO和1,2-HOPO具有最大的除铁剂潜力。
-
DIARYLMETHYLAMIDE DERIVATIVE HAVING ANTAGONISTIC ACTIVITY ON MELANIN-CONCENTRATING HORMONE RECEPTOR申请人:Banyu Pharmaceutical Co., Ltd.公开号:EP2272841A1公开(公告)日:2011-01-12[Problem] To provide a melanin-concentrating hormone receptor antagonist useful as a pharmaceutical agent for central diseases, circulatory diseases, and metabolic diseases. [Means for Resolution] Provided is a diarylmethylamide derivative represented by formula (I): Wherein R1a, R1b, R2a, R2b, R3a, and R3b independently represent a hydrogen atom or the like, R4 represents a hydrogen atom, C1-6 alkyl, or the like, R5 represents a hydrogen atom or the like, Z represents C1-6 alkyl or the like, or R4 and Z together form a 4- to 6-membered nitrogen-containing hetero ring, Y1 represents H or the like, Y2 represents H, or Y1 and Y2 together form - O-CH2-, W represents C, SO, or the like, Ar1 represents 6-membered aryl or the like, Ar2 represents 6-membered aryl or the like, and ring A represents a benzene ring, a pyridine ring, or the like.
-
Regioselective N‐Functionalization of Tautomerizable Heterocycles through Methyl Trifluoromethanesulfonate‐Catalyzed Substitution of Alcohols and Alkyl Group Migrations作者:Surajit Duari、Subrata Biswas、Arnab Roy、Srabani Maity、Abhishek Kumar Mishra、Aguinaldo R. Souza、Asma M. Elsharif、Nelson H. Morgon、Srijit BiswasDOI:10.1002/adsc.202101196日期:2022.2.15(MeOTf) was found to catalyze the reaction, which revealed the catalytic property of MeOTf. A mechanism was established through experiments as well as DFT calculations wherein the −OH group of alcohols were converted to the corresponding −OMe groups and in situ generated TfOH. The −OMe groups produced underwent TfOH catalyzed −X alkylation (X=O, S) of the heterocycles followed by −X- to −N-alkyl group
表征谱图
-
氢谱1HNMR
-
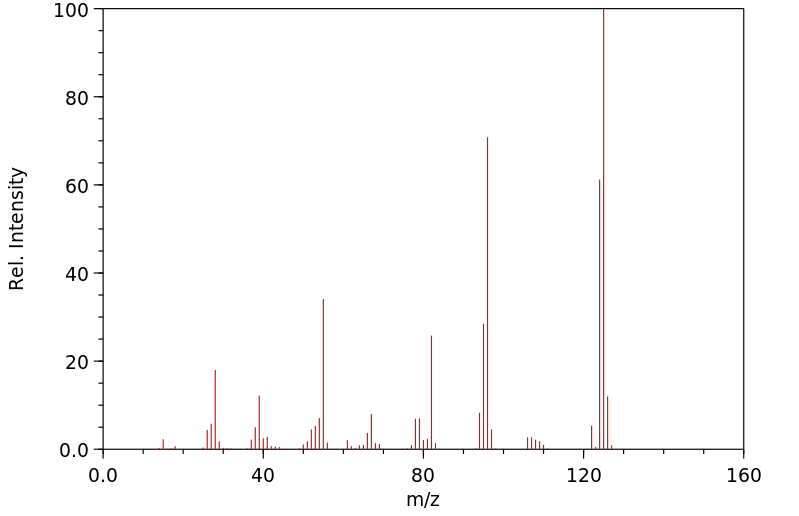
质谱MS
-
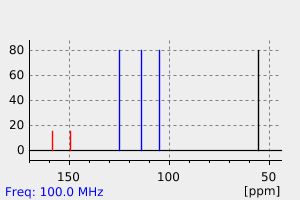
碳谱13CNMR
-
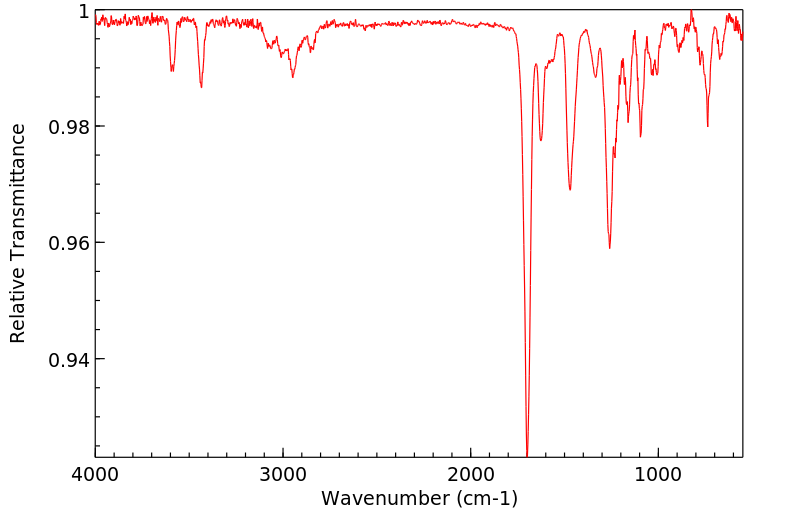
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-