(4S,5S)-(-)-2-乙基-4-羟甲基-5-苯基-2-恶唑啉 | 51594-33-3
中文名称
(4S,5S)-(-)-2-乙基-4-羟甲基-5-苯基-2-恶唑啉
中文别名
——
英文名称
(4S,5S)-(-)-2-ethyl-4-hydroxymethyl-5-phenyl-2-oxazoline
英文别名
(4S,5S)-2-Ethyl-4-hydroxymethyl-5-phenyl-1,3-oxazolin;((4S)-2-ethyl-5t-phenyl-4,5-dihydro-oxazol-4r-yl)-methanol;(-)-(4S,5S)-2-ethyl-5-phenyl-2-oxazoline-4-methanol;trans-(4S,5S)-2-ethyl-4-hydroxymethyl-5-phenyl-2-oxazoline;(4S,5S)-(-)-2-Ethyl-5-phenyl-2-oxazoline-4-methanol;[(4S,5S)-2-ethyl-5-phenyl-4,5-dihydro-1,3-oxazol-4-yl]methanol
CAS
51594-33-3
化学式
C12H15NO2
mdl
——
分子量
205.257
InChiKey
GZOYBTRDWOUZTG-JQWIXIFHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:15
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.42
-
拓扑面积:41.8
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2934999090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (4S,5S)-2-ethyl-4-methoxymethyl-5-phenyl-2-oxazoline 51594-37-7 C13H17NO2 219.283
反应信息
-
作为反应物:参考文献:名称:Asymmetric synthesis of R and S .alpha.-alkylalkanoic acids from metalation and alkylation of chiral 2-oxazolines摘要:DOI:10.1021/ja00418a041
-
作为产物:描述:(1S,2S)-(+)-2-氨基-1-苯基-1,3-丙二醇 、 丙酰胺 在 triethyloxonium fluoroborate 作用下, 生成 (4S,5S)-(-)-2-乙基-4-羟甲基-5-苯基-2-恶唑啉参考文献:名称:Meyers, A. I.; Hanagan, Mary Ann; Mazzu, Arthur L., Heterocycles, 1981, vol. 15, # 1, p. 361 - 367摘要:DOI:
-
作为试剂:描述:3,5-二甲氧基苯乙酮 在 吡啶 、 lithium aluminium tetrahydride 、 (4S,5S)-(-)-2-乙基-4-羟甲基-5-苯基-2-恶唑啉 作用下, 以 甲醇 、 水 为溶剂, 反应 2.5h, 生成 5-isopropyl-1,3-dimethoxybenzene参考文献:名称:手性,18 O标记的1-芳基乙酸乙酯的光溶剂分解的立体化学摘要:已经确定了(R)-(+)-1-(3,5-二甲氧基苯基)乙酸乙酯-醚-18O在甲醇-水和2,2,2-三氟乙醇中的光溶剂分解立体化学。在后一种溶剂中检测到离子对中间体。DOI:10.1016/0040-4039(81)80001-9
文献信息
-
Stereo-selective synthesis of 2-aryl-propionic acids of high optical申请人:——公开号:US05248815A1公开(公告)日:1993-09-28The present invention relates to a stereospecific chemical synthesis of optically pure enantiomers of 2-aryl-alkanoic acids, especially those of the biologically active (S)-aryl-propionic acids, in good chemical yields, useful for preparing large quantities thereof, and having a high optical purity.
-
Asymmetric synthesis of glycidic 2-oxazolines作者:Richard Liddell、Chris WhiteleyDOI:10.1039/c39830001535日期:——A stereospecific synthesis of 4S,5S-(–)-2-[(2R)-2,3-epoxy-2,3-dimethylbutyl]-4-methoxymethyl-5-phenyl-2-oxazoline via a Darzens condensation of 4S, 5S-(–)-2-(1-bromoethyl)-4-methoxymethyl-5-phenyl-2-oxazoline with propan-2-one is reported.
表征谱图
-
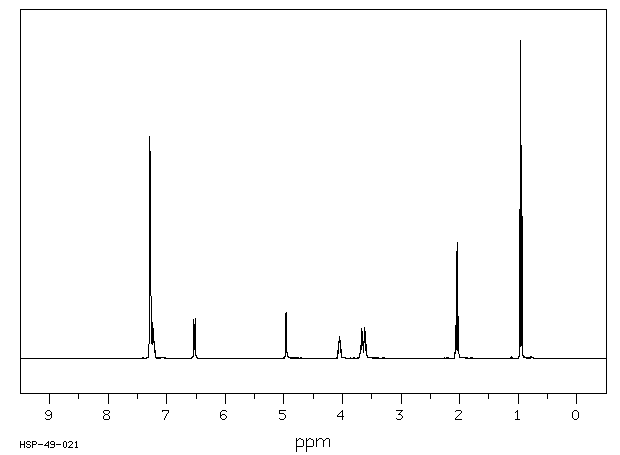
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
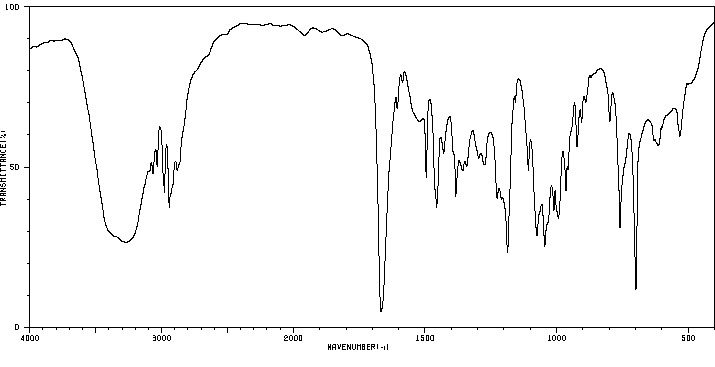
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫