3-苯乙烯基吡啶 | 2633-06-9
中文名称
3-苯乙烯基吡啶
中文别名
——
英文名称
(E)-3-(2-phenylvinyl)pyridine
英文别名
3-trans-styryl-pyridine;(E)-3-styrylpyridine;3-(2-Phenylethenyl)pyridine, (E)-;3-[(E)-2-phenylethenyl]pyridine
CAS
2633-06-9
化学式
C13H11N
mdl
——
分子量
181.237
InChiKey
RMSGACVDMOLUPL-CMDGGOBGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:80-81 °C
-
沸点:302.7±17.0 °C(Predicted)
-
密度:1.092±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:12.9
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-2-丙基(2-氨基苄基)氨基甲酸酯 (Z)-3-(β-bromovinyl)pyridine 125142-07-6 C7H6BrN 184.035 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (Z)-3-styrylpyridine 5097-90-5 C13H11N 181.237
反应信息
-
作为反应物:描述:3-苯乙烯基吡啶 在 palladium on activated charcoal 氢气 、 sodium amide 作用下, 以 乙醇 为溶剂, 反应 25.0h, 生成 2-Methyl-6-(2-phenylethyl)imidazo[1,2-a]pyridine参考文献:名称:抗溃疡药。1.取代的咪唑并[1,2-a]吡啶的胃抗分泌和细胞保护特性。摘要:描述了新型的抗溃疡剂,取代的咪唑并[1,2-a]吡啶。本发明的化合物既不是组胺(H 2)受体拮抗剂,也不是前列腺素类似物,但它们既具有胃分泌作用又具有细胞保护作用。胃抗分泌活性的机制可能涉及抑制H + / K + -ATPase酶。结构活性研究导致鉴定出3-(氰基甲基)-2-甲基-8-(苯甲氧基)咪唑并[1,2-a]吡啶,SCH 28080(27),已被选择用于进一步开发和临床评价。DOI:10.1021/jm00145a006
-
作为产物:参考文献:名称:Arylation of olefins by N-nitroso-N-arylacetamides under palladium(0) catalysis: a new precursor of arylpalladium species摘要:DOI:10.1021/jo00203a003
文献信息
-
醇供氢钯催化炔烃半还原选择性合成顺、反式烯烃的方法申请人:南通大学公开号:CN109776253B公开(公告)日:2022-07-15本发明提供了一种醇供氢钯催化炔烃半还原选择性合成顺、反式烯烃的方法,包括如下步骤:将TEOA、NaOAc、催化剂、醇与炔烃于有机溶剂中进行炔烃的还原反应,反应生成顺式烯烃;将配体、催化剂、醇与炔烃于有机溶剂中进行炔烃的还原反应,反应生成反式烯烃;还原反应的反应器为密封的耐压反应器,还原反应的温度为120~150℃,还原反应的时间为20~48h;催化剂的用量为炔烃摩尔用量的5~20%,醇的用量为炔烃摩尔用量的10~100倍;R,R‑DIPAMP的用量为炔烃摩尔用量的0.5~5倍。本发明中,催化剂体系具有极高的化学反应和立体选择性,能高产率合成顺式或者反式烯烃产物;催化体系对底物的普适性强,含各种官能团的炔烃都能高效地进行高选择性地还原反应。
-
Room-Temperature Suzuki-Miyaura Coupling of Heteroaryl Chlorides and Tosylates作者:Junfeng Yang、Sijia Liu、Jian-Feng Zheng、Jianrong Steve ZhouDOI:10.1002/ejoc.201200918日期:2012.11Suzuki–Miyaura coupling of heteroaryls is an important method for the preparation of compound libraries for medicinal chemistry and materials research. Although many catalysts have been developed, none of them have been generally applicable to the coupling reactions of heteroaryl chlorides and tosylates at room temperature. We discovered that a catalyst combination of Pd(OAc)2 and XPhos (2-dicyclohexylphosphanyl-2′杂芳基的 Suzuki-Miyaura 偶联是制备用于药物化学和材料研究的化合物库的重要方法。尽管已经开发了许多催化剂,但它们都没有普遍适用于杂芳基氯与甲苯磺酸酯在室温下的偶联反应。我们发现 Pd(OAc)2 和 XPhos(2-二环己基膦酰基-2',4',6'-三异丙基联苯)的催化剂组合可以有效地催化这些偶联。除了催化剂的选择,在含水醇溶剂中使用氢氧化物碱对于快速偶联也是必不可少的。这些条件促进了活性催化剂(XPhos)Pd0 的快速释放,并加速了催化循环中的金属转移。大多数杂芳基氯(31 个实例)和甲苯磺酸盐(17 个实例)的主要家族在室温下在几分钟到几小时内达到完全转化。该方法可以很容易地扩大规模以进行克级合成。此外,我们检查了整个反应中偶联伙伴的相对反应性。富电子杂芳基氯化物和甲苯磺酸盐的反应速度比缺电子杂芳基化合物慢,顺序为吲哚、吡咯呋喃、噻吩>吡啶。类似地,缺电子芳基硼酸的反应
-
Role of Mono-N-protected Amino Acid Ligands in Palladium(II)-Catalyzed Dehydrogenative Heck Reactions of Electron-Deficient (Hetero)arenes: Experimental and Computational Studies作者:Xuefeng Cong、Huarong Tang、Chao Wu、Xiaoming ZengDOI:10.1021/om400890p日期:2013.11.11We report here that mono-N-protected amino acids (MPAAs), an important environmentally compatible structural motif, enable acceleration of Pd(II)-catalyzed dehydrogenative Heck reactions between pyridines and electron-deficient arenes with simple alkenes, leading to diversely functionalized C3- or meta-selective alkenylated pyridines and benzenes via non-chelate-assisted C–H activation. A comprehensive我们在这里报告,单N保护的氨基酸(MPAAs),一种重要的环境相容性结构基序,能够加速Pd(II)催化的吡啶和电子不足的芳烃与简单烯烃之间的脱氢Heck反应,从而导致功能化的C3 -或meta通过非螯合物辅助的C–H活化选择性烯基化吡啶和苯。通过DFT计算进行的全面理论研究表明,MPAA配体的氨基支架通过最初的N–H活化容易地转化为X型配体,从而导致吡啶C–H裂解的活化障碍相对较低。然后,氨基的性质从X型配体转变为L型配体使得烯烃取代可以顺利进行,而羧基则可以形成分子内氢键,从而大大降低了碳pal触动的激活障碍。计算结果和动力学同位素效应测量结果支持了限速C–H活化,其机制涉及协同的金属化/去质子化途径,吸热值为31。
-
Palladium-Catalyzed Reductive Coupling Reaction of Terminal Alkynes with Aryl Iodides Utilizing Hafnocene Difluoride as a Hafnium Hydride Precursor Leading to <i>trans</i> -Alkenes作者:Keita Takahashi、Yohei Ogiwara、Norio SakaiDOI:10.1002/asia.201701775日期:2018.4.4Herein, we describe a reductive cross‐coupling of alkynes and aryl iodides by using a novel catalytic system composed of a catalytic amount of palladium dichloride and a promoter precursor, hafnocene difluoride (Cp2HfF2, Cp=cyclopentadienyl anion), in the presence of a mild reducing reagent, a hydrosilane, leading to a one‐pot preparation of trans‐alkenes. In this process, a series of coupling reactions
-
Electrochemical Aziridination of Internal Alkenes with Primary Amines作者:Maksim Ošeka、Gabriele Laudadio、Nicolaas P. van Leest、Marco Dyga、Aloisio de A. Bartolomeu、Lukas J. Gooßen、Bas de Bruin、Kleber T. de Oliveira、Timothy NoëlDOI:10.1016/j.chempr.2020.12.002日期:2021.1realizes this concept via an oxidative coupling between alkenes and primary alkylamines. The reaction is carried out in an electrochemical flow reactor leading to short reaction/residence times (5 min), high yields, and broad scope. At the cathode, hydrogen is generated, which can be used in a second reactor to reduce the aziridine, yielding the corresponding hydroaminated product. Mechanistic investigations
表征谱图
-
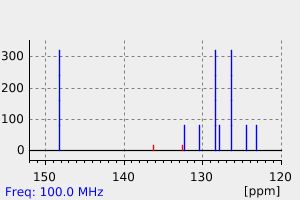
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫