2,4-二溴吡啶 | 58530-53-3
中文名称
2,4-二溴吡啶
中文别名
吡嗪2.3二羧酸
英文名称
2,4-dibromopyridine
英文别名
dibromo-2,4 pyridine
CAS
58530-53-3
化学式
C5H3Br2N
mdl
MFCD01859720
分子量
236.894
InChiKey
PCMMSLVJMKQWMQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:35-40 °C
-
沸点:238°C(lit.)
-
密度:2.059±0.06 g/cm3(Predicted)
-
闪点:>110℃
-
溶解度:溶于甲醇
-
稳定性/保质期:
避免接触氧化物
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:12.9
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R20/21/22
-
WGK Germany:1
-
危险品运输编号:UN 2811 6.1 / PGIII
-
海关编码:2933399090
-
危险标志:GHS05,GHS06
-
危险性描述:H301,H315,H318,H335
-
危险性防范说明:P261,P280,P301 + P310,P305 + P351 + P338
-
储存条件:将贮存于密闭容器中,并置于干燥、阴凉处保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2,4-Dibromopyridine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 2,4-Dibromopyridine
CAS number: 58530-53-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C5H3Br2N
Molecular weight: 236.9
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN2811 Class: 6.1 Packing group: III
Proper shipping name: TOXIC SOLIDS, ORGANIC, N.O.S. (2,4-Dibromopyridine)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2,4-Dibromopyridine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 2,4-Dibromopyridine
CAS number: 58530-53-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C5H3Br2N
Molecular weight: 236.9
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN2811 Class: 6.1 Packing group: III
Proper shipping name: TOXIC SOLIDS, ORGANIC, N.O.S. (2,4-Dibromopyridine)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4-二溴吡啶1-氧化物 2,4-dibromopyridine N-oxide 117196-08-4 C5H3Br2NO 252.893 2,3-二溴吡啶 2,3-dibromopyridine 13534-89-9 C5H3Br2N 236.894 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,4-二溴-3-甲基吡啶 2,4-dibromo-3-methylpyridine 128071-93-2 C6H5Br2N 250.92
反应信息
-
作为反应物:参考文献:名称:菲咯啉吲哚天然产物的模块合成。摘要:提出了一种高度简洁的菲咯啉吲哚全合成策略。芳基硼酸与邻-溴芳基N-甲基亚氨基二乙酸酯(MIDA)硼酸酯的一锅迭代Suzuki-Miyaura反应,然后与合适的吡啶溴化物提供的邻-氮杂-三联苯进行第二次Suzuki-Miyaura反应。随后使三键饱和,用甲磺酰氯处理,并还原所得的二氢吲哚并鎓环,得到六氢吲哚并酮。最终的钒催化氧化电环化仅在三个色谱柱分离操作中即可提供所需的生物碱。DOI:10.1021/acs.orglett.9b01397
-
作为产物:参考文献:名称:吡啶和其他杂环中甲硅烷基介导的卤素/卤素置换摘要:用溴三甲基硅烷加热将 2-氯吡啶转化为 2-溴吡啶,将 2-氯-6-甲基吡啶转化为 2-溴-6-甲基吡啶。2-氯吡啶和2-溴吡啶都得到相应的碘化合物。当用原位生成的碘三甲基硅烷处理时。尽管 3- 和 4- 氯吡啶是完全惰性的,但 2,4- 二氯吡啶在 2- 和 4- 位同时进行卤素/卤素交换。卤素置换仅在 2-位与 2,3-二氯吡啶和 2,5-二氯吡啶发生。与作为发生卤素交换的先决条件的 N-三甲基甲硅烷基吡啶鎓盐的中间体一致,2-氟吡啶和 2-氟-6-甲基吡啶和任何 2,6-二卤代吡啶都不发生反应。最后,溴/氯和碘/氯取代也可以用 2-或 4-氯喹啉、1-氯异喹啉、2-氯嘧啶、氯吡嗪和 2,3-二氯喹喔啉作为底物来完成。[在 SciFinder (R) 上]DOI:10.1002/1099-0690(200212)2002:24<4181::aid-ejoc4181>3.0.co;2-m
文献信息
-
Synthesis and biological evaluation of 4-(pyridin-4-oxy)-3-(3,3-difluorocyclobutyl)-pyrazole derivatives as novel potent transforming growth factor-β type 1 receptor inhibitors作者:Guofeng Xu、Yan Zhang、Hai Wang、Zhuang Guo、Xiaowei Wang、Xue Li、Shaohua Chang、Tianwen Sun、Zhuangzhuang Yu、Tianwei Xu、Liwen Zhao、Yazhou Wang、Wenying YuDOI:10.1016/j.ejmech.2020.112354日期:2020.7factor β (TGF-β) type 1 receptor (ALK5) provides a feasible approach for the treatment of fibrotic diseases and malignant tumors. In this study, we designed and synthesized a new series of 4-(pyridin-4-oxy)-3-(3,3-difluorocyclobutyl)-pyrazole derivatives, and evaluated biologically as TGF-β type 1 receptor inhibitors. The most potent compound 15r inhibited the ALK5 enzyme and NIH3T3 cell viability with IC50
-
Design, Synthesis, and Biological Evaluation of Novel Pyridine-Bridged Analogues of Combretastatin-A4 as Anticancer Agents作者:Shilong Zheng、Qiu Zhong、Madhusoodanan Mottamal、Qiang Zhang、Changde Zhang、Elise LeMelle、Harris McFerrin、Guangdi WangDOI:10.1021/jm500002k日期:2014.4.24A series of novel pyridine-bridged analogues of combretastatin-A4 (CA-4) were designed and synthesized. As expected, the 4-atom linker configuration retained little cytotoxicities in the compounds 2e, 3e, 3g, and 4i. Activities of the analogues with 3-atom linker varied widely depending on the phenyl ring substitutions, and the 3-atom linker containing nitrogen represents the more favorable linker设计并合成了一系列新型吡啶桥连的考布他汀-A4 (CA-4) 类似物。正如预期的那样,4 原子接头构型在化合物2e、3e、3g和4i 中几乎没有细胞毒性。具有 3 原子接头的类似物的活性因苯环取代而有很大差异,含氮的 3 原子接头代表更有利的接头结构。其中,三种类似物(4h、4s和4t)以与 CA-4 相当的方式在体内有效抑制细胞存活和生长、阻止细胞周期并阻断血管生成和脉管系统形成。叠加4H和微管蛋白的秋水仙碱结合口袋中的4s显示 CA- 4、4h和4s的结合姿势是相似的,正如竞争性结合试验所证实的,其中测量了配体替代微管蛋白结合的秋水仙碱的能力。结合数据与观察到的抗增殖和抑制血管生成的生物活性一致,但不能预测它们的抗微管蛋白聚合活性。
-
COMPOUNDS FOR THE TREATMENT OF PAIN申请人:Alkermes, Inc.公开号:US20190241524A1公开(公告)日:2019-08-08Provided herein are compounds that are useful in the treatment of pain in a subject.提供在本文件中的化合物可用于治疗主体的疼痛。
-
Pyrazolopyrimidines as therapeutic agents申请人:Abbott Laboratories公开号:US20020156081A1公开(公告)日:2002-10-24The present invention provides compounds of Formula I, 1 including pharmaceutically acceptable salts and/or prodrugs thereof, where G, R 2 , and R 3 are defined as described herein.本发明提供了公式I的化合物,包括其药学上可接受的盐和/或前药,其中G、R2和R3的定义如本文所述。
-
[EN] SUBSTITUTED IMIDAZOLES FOR THE INHIBITION OF TGF-BETA AND METHODS OF TREATMENT<br/>[FR] IMIDAZOLES SUBSTITUÉS POUR L'INHIBITION DE TGF-BÊTA ET MÉTHODES DE TRAITEMENT申请人:CLAVIUS PHARMACEUTICALS LLC公开号:WO2020041562A1公开(公告)日:2020-02-27This disclosure relates to low molecular weight substituted imidazoles that inhibit the TGF-b signaling pathway. More specifically, this disclosure relates to methods of using said imidazoles for the treatment of diseases related to the TGF-b signaling pathways including, but not limited to, atherosclerosis, Marfan syndrome, Loeys-Dietz syndrome, obesity, diabetes, multiple sclerosis, keratoconus, idiopathic pulmonary fibrosis, Alzheimer's Disease, chronic kidney disease, and scleroderma.
表征谱图
-
氢谱1HNMR
-
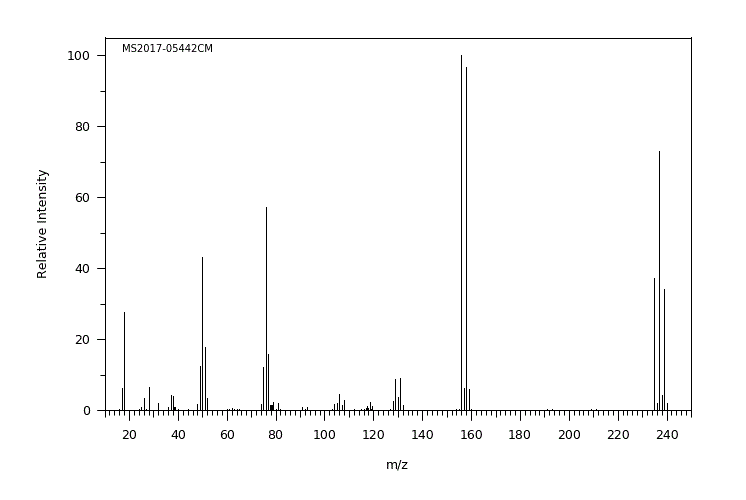
质谱MS
-
碳谱13CNMR
-
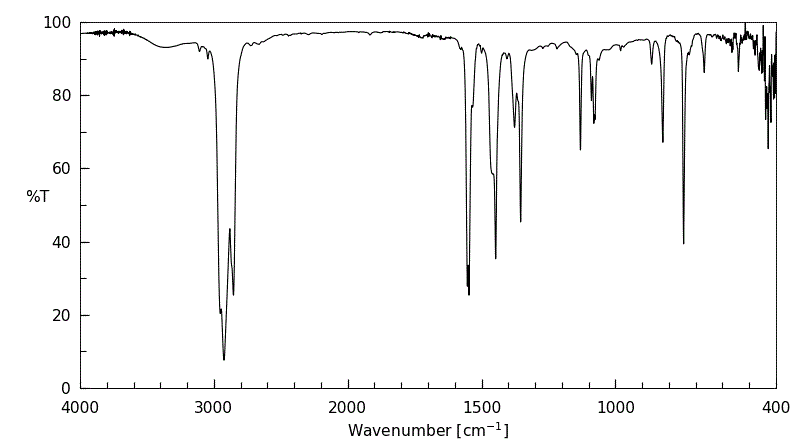
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-