dipropionamide | 6050-26-6
中文名称
——
中文别名
——
英文名称
dipropionamide
英文别名
Dipropionamid;Dipropionylamid;N-propanoylpropanamide
CAS
6050-26-6
化学式
C6H11NO2
mdl
——
分子量
129.159
InChiKey
GOJDSMIXPMMHPO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:239.22°C (rough estimate)
-
密度:1.1426 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:46.2
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Stereochemistry in trivalent nitrogen compounds. 43. Alkali metal complexation by thione sulfur in N-acylthioamides摘要:DOI:10.1021/ja00268a074
-
作为产物:描述:参考文献:名称:Otto; Troeger, Chemische Berichte, 1890, vol. 23, p. 760摘要:DOI:
文献信息
-
Palladium-catalyzed regiodivergent hydroaminocarbonylation of alkenes to primary amides with ammonium chloride作者:Bao Gao、Guoying Zhang、Xibing Zhou、Hanmin HuangDOI:10.1039/c7sc04054g日期:——the synthesis of primary amides has long been an elusive aim. Here, we report an efficient catalytic system which enables inexpensive NH4Cl to be utilized as a practical alternative to gaseous ammonia for the palladium-catalyzed alkene-hydroaminocarbonylation reaction. Through appropriate choice of the palladium precursors and ligands, either branched or linear primary amides can be obtained in good
-
Access to multi-functionalized oxazolines via silver-catalyzed heteroannulation of enamides with sulfoxonium ylides作者:Rui-Hua Liu、Qi-Chao Shan、Ya Gao、Teck-Peng Loh、Xu-Hong HuDOI:10.1016/j.cclet.2020.10.007日期:2021.4efficient Ag-catalyzed [4 + 1] heteroannulation reaction of enamides with α-carbonyl sulfoxonium ylides. The diastereoselective transformation provides a practical access to a diverse range of multi-functionalized oxazoline derivatives. The synthetic utility of the resultant tetra-substituted oxazolines is further demonstrated by a series of useful manipulations into valuable building blocks of pharmaceutical
-
Studies on Nitrile Salts. I. Dimerization of Nitriles Having α-Hydrogen in the Presence of Hydrogen Chloride作者:Shozo Yanagida、Tetsuo Fujita、Masataka Ohoka、Ichiro Katagiri、Saburo KomoriDOI:10.1246/bcsj.46.292日期:1973.1chloroacetonitrile was confirmed to be N-(α,α,β-trichloroethyl)chloroacetamidine hydrochloride. The scope and limitations of the dimerization reaction of nitriles having α-hydrogen with HCl were studied. Most nitriles having α-hydrogen react with HCl to give N-(α-chloroalkenyl)alkylamidine hydrochlorides (3). Their hydrolysis to diacylamines (4) was also investigated.
-
PROCESS FOR ALKENYLATING CARBOXAMIDES申请人:Staffel Wolfgang公开号:US20090131657A1公开(公告)日:2009-05-21The present invention relates to a process for preparing N-(1-alkenyl)carboxamides of the formula I, which comprises reacting a carboxamide of the formula II with an alkyne of the formula III in the presence of a catalyst selected from among carbonyl complexes, halides and oxides of rhenium, manganese, tungsten, molybdenum, chromium and iron.
-
Autoxidation of N-alkylamides. Part I. N-Acylamides as oxidation products作者:M. V. Lock、B. F. SagarDOI:10.1039/j29660000690日期:——Products of the thermal and photosensitised autoxidation of N-alkyl- and NN-dialkyl-amides have been identified. N-n-Alkylamides yield principally N-acylamides, primary amides, and N-formylamides, as a result of initial abstraction of a hydrogen atom from the carbon adjacent to nitrogen. Formation of N-formylamides, and of N-acylamides from N-s-alkylamides, involves C(1)–C(2) bond scission in an N-alkyl已经鉴定了N-烷基-和NN-二烷基酰胺的热和光敏自氧化的产物。Ñ -n烷基酰胺得到主要Ñ -acylamides,伯酰胺,和Ñ -formylamides,如从与氮相邻碳氢原子的初始抽象的结果。的形成Ñ -formylamides,以及Ñ从-acylamides Ñ -s-三烷基酰胺,涉及Ç (1) -C (2)键的断裂中ñ -烷基。NN的氧化-二烷基酰胺遵循相似的模式。给出了89种酰胺的气相色谱-液相色谱保留数据。
表征谱图
-
氢谱1HNMR
-
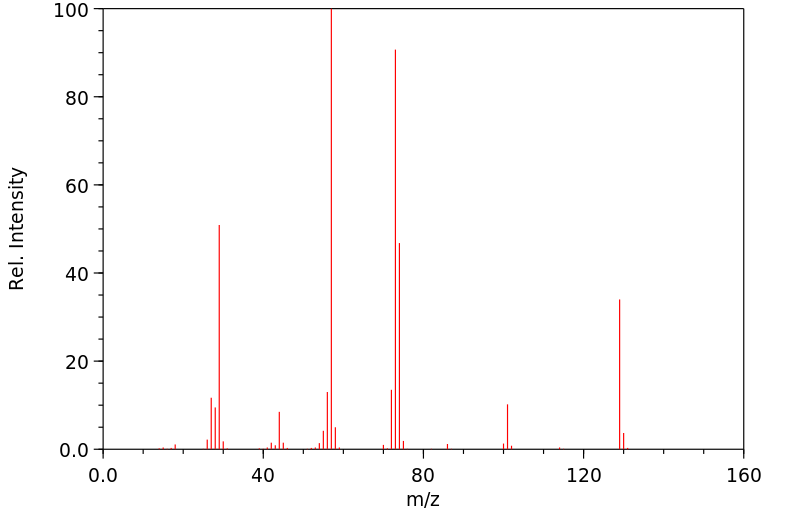
质谱MS
-
碳谱13CNMR
-
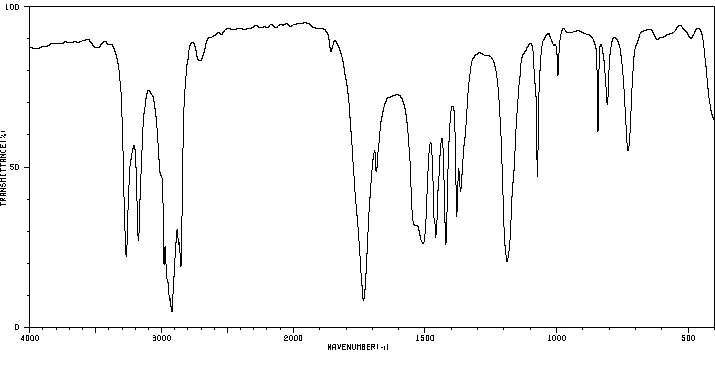
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸