7-乙酰氧基-4-溴甲基香豆素 | 2747-04-8
中文名称
7-乙酰氧基-4-溴甲基香豆素
中文别名
4-溴甲基-7-乙酸香豆素;7-乙酰氧基-4-溴甲基香豆素[用于高效液相色谱标记]
英文名称
7-acetoxy-4-bromomethyl-2H-1-benzopyran-2-one
英文别名
7-acetoxy-4-(bromomethyl)coumarin;7-acetoxy-4-bromomethylcoumarin;7-acetoxy-4-bromomethyl-chromen-2-one;7-acetoxy-4-bromomethyl-coumarin;7-Acetoxy-4-brommethyl-cumarin;4-Bromomethyl-7-acetoxycoumarin;[4-(bromomethyl)-2-oxochromen-7-yl] acetate
CAS
2747-04-8
化学式
C12H9BrO4
mdl
MFCD00010707
分子量
297.105
InChiKey
YDJFMNSSDOXBSR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:180-182°C
-
沸点:414.4±45.0 °C(Predicted)
-
密度:1.589±0.06 g/cm3(Predicted)
-
溶解度:溶于氯仿、乙酸乙酯
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S22,S26,S27,S36/37/39,S45
-
危险类别码:R34
-
危险品运输编号:1759
-
海关编码:2932209090
-
包装等级:III
-
危险类别:8
-
储存条件:存放于阴凉干燥处。
SDS
7-Acetoxy-4-bromomethylcoumarin [for HPLC Labeling] Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 7-Acetoxy-4-bromomethylcoumarin [for HPLC Labeling]
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS
Corrosive to metals Category 1
HEALTH HAZARDS
Skin corrosion/irritation Category 1B
Category 1
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols
Signal word Danger
Hazard statement May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements
Keep only in original container.
[Prevention]
Do not breathe.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
[Response]
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance
Substance/mixture:
Labeling]
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Component(s): 7-Acetoxy-4-bromomethylcoumarin [for HPLC Labeling]
Percent: >98.0%(LC)(T)
CAS Number: 2747-04-8
Chemical Formula: C12H9BrO4
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (P3 filter respirator for toxic particles). Keep
protective equipment and people away from and upwind of spill/leak. Entry to non-involved personnel should
emergency procedures: be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a local exhaust if dust or aerosol will be
generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Use corrosive resistant equipment.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed. Keep only in original container.
Labeling]
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
White - Slightly pale yellow
Color:
Odor: No data available
pH: No data available
Melting point/freezing point:182 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Conditions to avoid: Light-sensitive, Moisture-sensitive
Incompartible materials: oxidizing agents, bases
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Labeling]
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
8: Corrosive.
Hazards Class:
UN-No: 3261
Corrosive solid, acidic, organic, n.o.s.
Proper shipping name:
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 7-Acetoxy-4-bromomethylcoumarin [for HPLC Labeling]
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS
Corrosive to metals Category 1
HEALTH HAZARDS
Skin corrosion/irritation Category 1B
Category 1
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols
Signal word Danger
Hazard statement May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements
Keep only in original container.
[Prevention]
Do not breathe.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
[Response]
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance
Substance/mixture:
Labeling]
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Component(s): 7-Acetoxy-4-bromomethylcoumarin [for HPLC Labeling]
Percent: >98.0%(LC)(T)
CAS Number: 2747-04-8
Chemical Formula: C12H9BrO4
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (P3 filter respirator for toxic particles). Keep
protective equipment and people away from and upwind of spill/leak. Entry to non-involved personnel should
emergency procedures: be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a local exhaust if dust or aerosol will be
generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Use corrosive resistant equipment.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed. Keep only in original container.
Labeling]
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
White - Slightly pale yellow
Color:
Odor: No data available
pH: No data available
Melting point/freezing point:182 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Conditions to avoid: Light-sensitive, Moisture-sensitive
Incompartible materials: oxidizing agents, bases
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Labeling]
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
8: Corrosive.
Hazards Class:
UN-No: 3261
Corrosive solid, acidic, organic, n.o.s.
Proper shipping name:
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 7-乙酰氧基-4-甲基香豆素 4-methylumbelliferyl acetate 2747-05-9 C12H10O4 218.209 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(溴甲基)-7-羟基苯并吡喃-2-酮 7-hydroxy-4-bromomethylcoumarin 161798-25-0 C10H7BrO3 255.068 7-羟基-4-(羟基甲基)-2H-色烯-2-酮 7-hydroxy-4-(hydroxymethyl)-2H-chromen-2-one 151889-83-7 C10H8O4 192.171 —— 4-(azidomethyl)-7-hydroxy-2H-chromen-2-one 866560-73-8 C10H7N3O3 217.184 —— 7-acetoxy-4-(2-nitroimidazol-1-ylmethyl)-2H-1-benzopyran-2-one 137046-44-7 C15H11N3O6 329.269
反应信息
-
作为反应物:描述:7-乙酰氧基-4-溴甲基香豆素 在 sodium azide 作用下, 以 乙醇 、 水 为溶剂, 反应 11.0h, 以56%的产率得到4-(azidomethyl)-7-hydroxy-2H-chromen-2-one参考文献:名称:Synthesis and single enzyme activity of a clicked lipase–BSA hetero-dimer摘要:点击化学被用于构建一种新型的脂肪酶–BSA异二聚体,其中后者蛋白质作为基脚,使酶能够锚定在表面上,便于进行单酶研究。DOI:10.1039/b516551b
-
作为产物:描述:7-乙酰氧基-4-甲基香豆素 以60%的产率得到7-乙酰氧基-4-溴甲基香豆素参考文献:名称:US6133460摘要:公开号:
文献信息
-
Halogen‐Mediated Membrane Transport: An Efficient Strategy for the Enhancement of Cellular Uptake of Synthetic Molecules作者:Harinarayana Ungati、Vijayakumar Govindaraj、Chithra R. Nair、Govindasamy MugeshDOI:10.1002/chem.201806122日期:——biological molecules require specific membrane transporters and channel proteins that control the traffic of these molecules into and out of the cell. This work reports that the introduction of halogen atoms into a series of fluorescent molecules remarkably enhances their cellular uptake, and that their transport can be increased to more than 95 % by introducing two iodine atoms at appropriate positions. The哺乳动物细胞对荧光探针和治疗剂的吸收不良是生物学应用中的一个主要问题,涉及从荧光成像到活细胞内药物传递的各种领域。尽管气态分子(例如氧气和二氧化碳),疏水性物质(例如苯)以及小的极性但不带电荷的分子(例如水和乙醇)可以通过简单的被动扩散穿过细胞质膜,但许多合成分子和生物分子都需要特定的膜转运蛋白和控制这些分子进出细胞的通道蛋白。这项工作报告说,将卤素原子引入一系列荧光分子中可以显着增强其细胞摄取,并且通过在适当位置引入两个碘原子,可以将其转运提高到95%以上。当分子中存在碘原子时,荧光团的性质在细胞摄取中并不起主要作用,因为带有萘二甲酰亚胺,香豆素,BODIPY和pyr部分的化合物显示出相似的摄取。有趣的是,引入带有两个羟乙基硫基的马来酰亚胺基荧光团使分子能够穿过质膜和核膜,碘原子的存在进一步增强了跨这两个膜的传输。总的来说,这项研究提供了增强哺乳动物细胞对有机分子吸收的一般策略。引入带有
-
An Efficient Procedure for the 1-Alkylation of 2-Nitroimidazoles and the Synthesis of a Probe for Hypoxia in Solid Tumours作者:Anthony Long、John Parrick、Richard J. HodgkissDOI:10.1055/s-1991-26552日期:——The N-alkylation of 2-nitroimidazole through its 'naked' anion, formed from its alkali metal salts in the presence of crown ethers and in homogeneous solution, is shown to be a useful preparative procedure. The reactions of α,Ï-dihaloalkanes can be controlled to afford either the mono- or diheteroarylalkane. The procedure has been used to alkylate theophylline and to prepare a compound of use as a probe to identify, locate and quantify hypoxia in sections from solid tumours.
-
COMPOSITIONS AND METHODS FOR TREATMENT OF VIRAL DISEASES申请人:Johansen Lisa M.公开号:US20100009970A1公开(公告)日:2010-01-14The present invention features compositions, methods, and kits useful in the treatment of viral diseases. In certain embodiments, the viral disease is caused by a single stranded RNA virus, a flaviviridae virus, or a hepatic virus. In particular embodiments, the viral disease is viral hepatitis (e.g., hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E) and the agent or combination of agents includes sertraline, a sertraline analog, UK-416244, or a UK-416244 analog. Also featured are screening methods for identification of novel compounds that may be used to treat a viral disease.
-
Preparation and Molecular Recognition of SERS Probe Based on Gold Nanoparticles Constructed from PEG–Oligoamine Copolymer Possessing a Coumarin Group between PEG and Oligoamine作者:Hitoshi Furusho、Motoi Oishi、Tetsuo Kishi、Atsuo Yasumori、Yukio NagasakiDOI:10.1246/cl.2010.52日期:2010.1.5Novel gold nanoparticles (GNPs) as a SERS probe were prepared using two types of functional PEG/oligoamine polymers possessing biotin as biotag molecules at the PEG end or a coumarin moiety as a Raman probe molecule between the PEG and oligoamine segments. Eventually, the GNPs showed excellent dispersion stability under physiological conditions, as well as remarkable SERS signal resulting from molecular recognition between streptavidin and the biotin tag, even in the presence of bovine serum albumin (BSA).
-
Psoralens for pathogen inactivation申请人:Cerus Corporation公开号:US20030082510A1公开(公告)日:2003-05-01Psoralen compound compositions are synthesized which have primaryamino substitutions on the 3-, 4-, 5-, and 8-positions of the psoralen, which yet permit their binding to nucleic acid of pathogens. Reaction conditions that photoactivate these psoralens result in the inactivation of pathogens which contain nucleic acid. The compounds show similar activity in test systems to 4′ and 5′ derivatives of psoralen useful for inactivation of pathogens in blood products. In addition to the psoralen compositions, the invention contemplates such inactivating methods using the new psoralens.
表征谱图
-
氢谱1HNMR
-
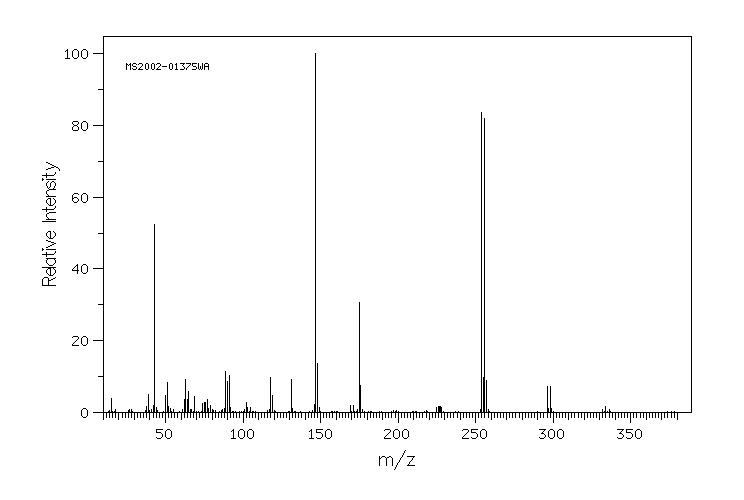
质谱MS
-
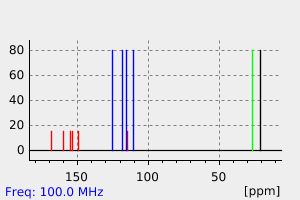
碳谱13CNMR
-
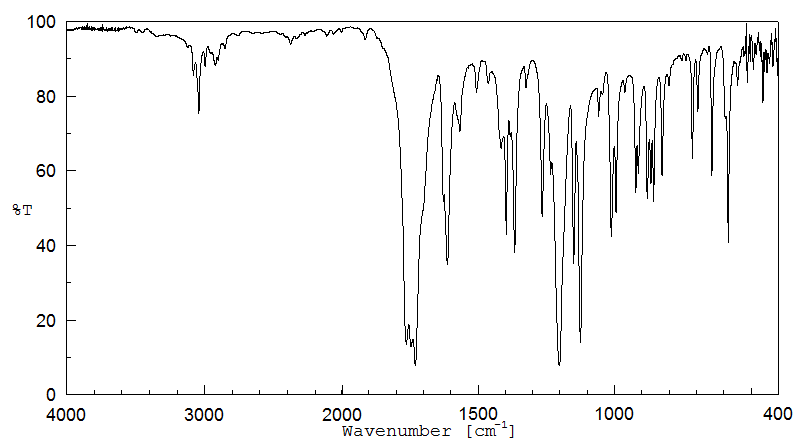
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄皮香豆精
黄木亭
黄曲霉素P2
黄曲霉素P1
黄曲霉素G2-13C17-同位素
黄曲霉素G2
黄曲霉素G1-13C17-同位素
黄曲霉素B2-13C17-同位素
黄曲霉素B1-13C17-同位素
黄曲霉素B1 8,9-环氧化物
黄曲霉素 G1
黄曲霉毒醇Ⅱ
黄曲霉毒醇M1
黄曲霉毒醇A
黄曲霉毒素M2
黄曲霉毒素M1-(O-羧甲基)肟
黄曲霉毒素G2a
黄曲霉毒素G19,10-环氧化物
黄曲霉毒素B2
黄曲霉毒素B1二氯化物
黄曲霉毒素B1-8,9-二氯化物
黄曲霉毒素B1-(O-羧甲基)肟
黄曲霉毒素 Q1
黄曲霉毒素 M1
黄曲霉毒素 B2
黄曲霉毒素 B1
黄曲霉毒素
香豆霉素
香豆素6H
香豆素545T
香豆素545
香豆素525
香豆素343甲酯
香豆素338
香豆素314T
香豆素175
香豆素152
香豆素106
香豆素-D4
香豆素-6-磺酰氯
香豆素-6-甲醛
香豆素-5-氧丁酸
香豆素-4-乙酸
香豆素-3腈
香豆素-35
香豆素-3-羧酸酸酐
香豆素-3-羧酸琥珀酰亚胺酯
香豆素-3-羧酸乙酯
香豆素-3-羧酸
香豆素-3-甲酰氯