2,5-二氧代吡咯烷-1-羧酸苄酯 | 75315-63-8
中文名称
2,5-二氧代吡咯烷-1-羧酸苄酯
中文别名
——
英文名称
N-(benzyloxycarbonyl)succinimide
英文别名
benzyloxycarbonyl succinimide;Benzyl 2,5-dioxopyrrolidine-1-carboxylate
CAS
75315-63-8
化学式
C12H11NO4
mdl
——
分子量
233.224
InChiKey
UTZLYIFQYGQUPA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:63.7
-
氢给体数:0
-
氢受体数:4
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-Cbz-吡咯烷 1-benzyloxycarbonylpyrrolidine 25070-74-0 C12H15NO2 205.257 (2S)-N-苄氧羰基-2-吡咯烷甲醛 (S)-2-formylpyrrolidine-1-carboxylic acid benzyl ester 71461-30-8 C13H15NO3 233.267 3-CBZ-6-氧杂-3-氮杂二环[3.1.0]己烷 benzyl 6-oxa-3-azabicyclo[3.1.0]hexane-3-carboxylate 31865-25-5 C12H13NO3 219.24 —— benzyl 4-(2-((tert-butoxycarbonyl)(methyl)amino)ethyl)piperidine-1-carboxylate 171049-38-0 C21H32N2O4 376.496 Z-L-脯氨醇 N-Cbz-Prolinol 6216-63-3 C13H17NO3 235.283 —— (2-amino-ethyl)-ethyl-carbamic acid benzyl ester 126955-78-0 C12H18N2O2 222.287 —— (S)-1-benzyloxycarbonyl-2-azetidinemethanol 186593-56-6 C12H15NO3 221.256 N-苯甲氧基甲酰基-1,2,5,6-四氢吡啶 1-benzyloxycarbonyl-1,2,3,6-tetrahydropyridine 66207-23-6 C13H15NO2 217.268 —— benzyl diallylcarbamate 25070-76-2 C14H17NO2 231.294 N-Cbz-N-甲基乙二胺 (2-aminoethyl)-methylcarbamic acid,benzyl ester 19023-94-0 C11H16N2O2 208.26 3-吡咯烷-1-甲酸苄酯 1-carboxybenzyl-3-pyrrolidine 31970-04-4 C12H13NO2 203.241 1,4,7,10-四氮杂环十二烷-1,7-二羧酸二苄酯 1,4,7,10-tetraaza-cyclododecane-1,7-dicarboxylic acid dibenzyl ester 162148-45-0 C24H32N4O4 440.542 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Unexpected diastereoselectivity in AD of an l-proline-derived 1,1-disubstituted alkene摘要:Asymmetric dihydroxylation of the L-proline-derived 1,1-disubstituted alkene 5 catalysed by either (DHQ)(2)PHAL or (DHQD)(2)PHAL unexpectedly leads to preference for the same diastereomer 7, both reactions proceeding with apparently matching double diastereoselectivity. In contrast, AD using the analogous (DHQ)(2)PYR or (DHQD)(2)PYR ligands leads to preferences for diols 7 or 8 respectively. (C) 1998 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4039(97)10695-5
-
作为产物:参考文献:名称:N-酰基和N-氨基甲酰基琥珀酰亚胺的合成、X射线晶体学和反应摘要:摘要 通过琥珀酰亚胺与酰氯的酰化或羧酸的二氯乙烷 (EDC) 偶联制备了一系列 N-酰基和 N-氨基甲酰基琥珀酰亚胺。确定了 N-苯甲酰基和 Np-硝基苯甲酰基琥珀酰亚胺的 X 射线晶体结构。N-酰基琥珀酰亚胺可有效地酰化伯胺、仲胺和芳香胺。补充材料可用于本文。转至出版商的 Synthetic Communications® 在线版以查看免费的补充文件。图形概要DOI:10.1080/00397911.2012.690061
-
作为试剂:描述:参考文献:名称:一种(S)-1-(苄氧羰基)-5-氧代吡咯烷-2-甲酸的制备方法摘要:本发明公开了一种(S)‑1‑(苄氧羰基)‑5‑氧代吡咯烷‑2‑甲酸的制备方法,主要解决原工艺中的复杂性,周期长,成本高、纯度低等技术问题,本发明具体包括以下步骤:第一步,由L‑谷氨酸和苄氧羰基供体制备得N‑苄氧羰基‑L‑谷氨酸,第二步,将N‑苄氧羰基‑L‑谷氨酸进行分子内缩合环化制得N‑苄氧羰基‑L‑谷氨酸粗品;第三步,将N‑苄氧羰基‑L‑谷氨酸粗品和有机胺碱混合,利用产品在溶剂中的溶解度制得其有机胺盐式,第四将N‑苄氧羰基‑L‑谷氨酸有机胺盐式脱盐后制备得(S)‑1‑(苄氧羰基)‑5‑氧代吡咯烷‑2‑甲酸。本发明制备得到高纯度的产品,收率和品质都得到了较大的提高。公开号:CN113321606A
文献信息
-
Total Syntheses of Conformationally Constrained Didemnin B Analogues. Replacements of <i>N</i>,<i>O</i>-Dimethyltyrosine with <scp>l</scp>-1,2,3,4-Tetrahydroisoquinoline and <scp>l</scp>-1,2,3,4-Tetrahydro-7-methoxyisoquinoline作者:James E. Tarver、Amy J. Pfizenmayer、Madeleine M. JoulliéDOI:10.1021/jo0105991日期:2001.11.1The design and synthesis of two conformationally constrained analogues of didemnin B are described. The [N,O-Me(2)Tyr(5)]residue of didemnin B was replaced with L-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) and L-1,2,3,4-tetrahydro-7-methoxyisoquinoline-3-carboxylic acid (MeO-Tic), which mimic the N,O-dimethylated tyrosine while constraining the conformation of the molecule. Preliminary描述和设计了两种构型受约束的二氢蝶呤B的类似物。豆双宁B的[N,O-Me(2)Tyr(5)]残基被L-1,2,3,4-四氢异喹啉-3-羧酸(Tic)和L-1,2,3取代, 4-四氢-7-甲氧基异喹啉-3-羧酸(MeO-Tic),可模仿N,O-二甲基化的酪氨酸,同时限制分子的构象。初步结果表明[N,O-Me(2)Tyr(5)]残基的构象与Tic替代所施加的构象非常匹配。
-
Pyrimidine Derivatives Which Are Antagonists Of Vitronectin Receptor申请人:Lefrancois Jean-Michel公开号:US20080058348A1公开(公告)日:2008-03-06A subject of the invention is the compounds of formula (I); in which R 1 , R 2 , R 3 , R 4 and R have the meanings indicated in the description, their preparation process, their use as medicaments having an antagonist activity on the vitronectin receptor and the pharmaceutical compositions containing them.本发明的主题是公式(I)的化合物;其中R1、R2、R3、R4和R具有说明书中指出的含义,它们的制备过程,它们作为对玻连蛋白受体具有拮抗活性的药物的使用以及包含它们的药物组合物。
-
Compositions Comprising Enzyme-Cleavable Oxycodone Prodrug申请人:Jenkins Thomas E.公开号:US20120178773A1公开(公告)日:2012-07-12The embodiments provide Compound KC-7, N-1-[(S)-2-(oxycodone-6-enol-carbonyl-methyl-amino)-2-carbonyl-sarcosine-ethyl amine]-arginine-glycine-acetate, or acceptable salts, solvates, and hydrates thereof. The present disclosure also provides compositions, and their methods of use, where the \compositions comprise a prodrug, Compound KC-7, that provides controlled release of oxycodone. Such compositions can optionally provide a trypsin inhibitor that interacts with the enzyme that mediates the controlled release of oxycodone from the prodrug so as to attenuate enzymatic cleavage of the prodrug.
-
Active agent prodrugs with heterocyclic linkers申请人:Jenkins Thomas E.公开号:US09139612B2公开(公告)日:2015-09-22The embodiments provide prodrug compounds of Formulae I-XVII. The present disclosure also provides compositions, and their methods of use, where the compositions comprise a prodrug compound of Formulae I-XVII that provides controlled release of an active agent. Such compositions can optionally provide a trypsin inhibitor that interacts with the enzyme that mediates the controlled release of an active agent from the prodrug so as to attenuate enzymatic cleavage of the prodrug.本发明的实施例提供了I-XVII号化合物的前药。本公开还提供了组合物及其使用方法,其中所述组合物包含能控制释放活性剂的前药化合物I-XVII。这样的组合物可以选择性地提供一种胰蛋白酶抑制剂,该抑制剂与介导前药控制释放活性剂的酶相互作用,从而减弱前药的酶切裂。
-
[EN] N-METHYL AMINO ACIDS<br/>[FR] ACIDES N-METHYL AMINES申请人:UBIQUITOUS TECHNOLOGIES PTY LT公开号:WO2004007427A1公开(公告)日:2004-01-22The present invention relates to a compound of formula (I) or (II), processes for preparing them, peptides including them and kits involving them.这项发明涉及到式(I)或(II)的化合物,制备它们的方法,包括它们的肽和涉及它们的试剂盒。
表征谱图
-
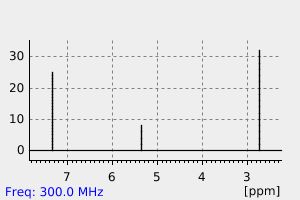
氢谱1HNMR
-
质谱MS
-
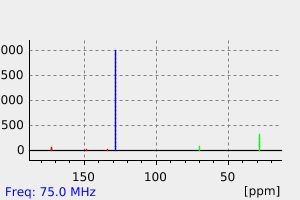
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫